Trending...
- Crunchbase Ranks Phinge Founder & CEO Robert DeMaio #1 Globally. Meet him in Las Vegas-Week of CES to Learn About Netverse, Patented App-less Platform
- Contracting Resources Group Receives 2025 HIRE Vets Platinum Medallion Award from the U.S. Department of Labor
- VSee Health (N A S D A Q: VSEE) Secures $6.0M At-Market Investment, Accelerates Expansion as Revenues Surge
NRXP: Potential Acquisition and Financing Agreements for $30 Million in Currently-Operating Interventional Psychiatry Clinics.
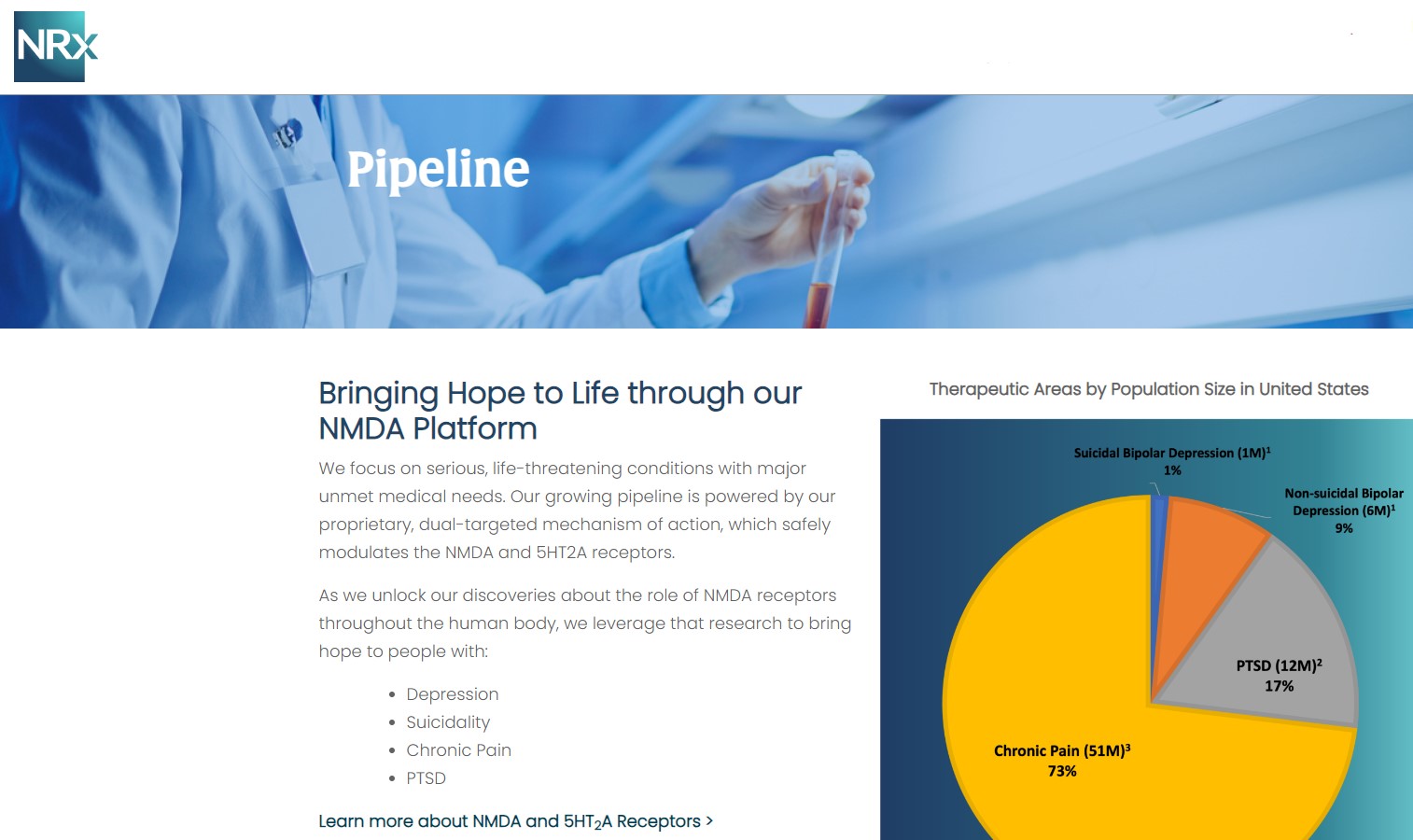
MIAMI - Michimich -- NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Potential Acquisition and Financing Agreements for $30 Million in Currently-Operating Interventional Psychiatry Clinics
On August 26th NRXP announced signing a non-binding Term Sheet for non-dilutive, nonconvertible debt acquisition funding of its first interventional psychiatry clinics (ketamine clinic acquisitions), together with the signing of a Term Sheet for five currently operational clinics in the Western United States. In addition to the currently-signed non-binding Term Sheet, NRXP has been offered non-binding lending commitments it believes are sufficient to assemble/acquire a network of operational clinics with revenues in excess of $100 million. NRXP anticipates potential operations in the United States, France, and the United Kingdom.
More on Michimich.com
This non-dilutive acquisition funding is in addition to the over $60 million in potential equity funding previously offered upon public listing of HOPE Therapeutics shares on a public exchange. Additional information to be presented by NRXP at upcoming HC Wainwright Annual Global Investment Conference in New York, September 9-11, 2024.
NRXP has formed partnerships to license clinical trial data from a French Government-funded trial and two NIH-funded trials all of which demonstrate efficacy of racemic Intravenous ketamine against depression and two of which demonstrate statistically significant benefit vs suicidality.
In March 2024 NRXP demonstrated the formulation of a pH neutral patentable form of IV ketamine that it anticipates will have widespread applicability both in treatment of depression and chronic pain.
NRXP presented final data from the recently completed phase 2b/3 trial of NRX-101 in suicidal bipolar depression4 at the American Society of Clinical Psychopharmacology's annual meeting. These data demonstrated a significantly improved safety profile versus the standard of care, as demonstrated by a clinically significant reduction in akathisia (P=0.025) and time to sustained remission from suicidality (P=0.05). Akathisia is an adverse event seen with antidepressant medications considered by many experts to be a precursor to suicide. Given the vital need for safer medications in this at-risk population, we plan to submit an NDA for Accelerated Approval to the US FDA for treatment of bipolar depression patients with suicidality or akathisia, based on these data as well as additional data from our STABIL-B5 trial.
HOPE Therapeutics, is an NXRP subsidiary that will focus on the delivery of advance psychiatric treatments, including ketamine-focused treatment for depression and suicidality. Unlike the core business of NRXP, that is focused on biotechnology Research and Development, HOPE is organized around consolidating existing best-in-class clinics into a nationwide network. This has been done previously and without much success with clinics that are not necessarily psychiatrist-led. With Hope ownership as an asset of NRXP, this will further strengthen the NRXP balance sheet and aims to further enhance shareholder value.
More on Michimich.com
NRX-101 for Treatment of Chronic Pain:
In 2023, NRXP licensed US Patent 8,653,120 for the use of DCS in chronic pain and filed a now-accepted Investigational New Drug (IND) application with the FDA to initiate commercial drug development of NRX-101 in chronic pain. Data lock has now been achieved in a 200-person randomized prospective trial funded by the US DOD.
During Q3 2023, NRXP tested NRX-101 and its components against resistant pathogens that appear on the Congressionally mandated Qualified Infectious Disease Product (QIDP) list and proved in vitro effectiveness against antibiotic-resistant E. coli, Pseudomonas, and Acinetobacter. Accordingly, NRXP was granted QIDP designation, Fast Track Designation, and Priority Review by the US FDA in January 2024.
Should NRX-101 succeed in clinical trials, NRXP will consider developing a follow-on product that is anticipated to achieve another 20 years of patent exclusivity.
NRXP recently announced data demonstrating that NRX-101 does not compromise the intestinal microbiome, unlike common antibiotics including Clindamycin and Ciprofloxacin. Should these findings be documented in human patients, NRX-101 would represent the only treatment for cUTI that does not cause C. Difficile infection.
Additionally, should NRXP or its partners succeed in serving 10% of the cUTI market, NRXP believes that the revenue from NRX-101 has the potential to be hundreds of million annually, based on 3 million cases per year in the US and potential pricing of over $3,500/course of therapy.
Media Contact
Company Name: NRx Pharmaceuticals, Inc.
Contact Person: Matthew Duffy, Chief Business Officer
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Country: United States
Website: https://www.nrxpharma.com/
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Potential Acquisition and Financing Agreements for $30 Million in Currently-Operating Interventional Psychiatry Clinics
On August 26th NRXP announced signing a non-binding Term Sheet for non-dilutive, nonconvertible debt acquisition funding of its first interventional psychiatry clinics (ketamine clinic acquisitions), together with the signing of a Term Sheet for five currently operational clinics in the Western United States. In addition to the currently-signed non-binding Term Sheet, NRXP has been offered non-binding lending commitments it believes are sufficient to assemble/acquire a network of operational clinics with revenues in excess of $100 million. NRXP anticipates potential operations in the United States, France, and the United Kingdom.
More on Michimich.com
- A New Soul Album: Heart Of Kwanzaa, 7-Day Celebration
- Allegiant Management Group Named 2025 Market Leader in Orlando by PropertyManagement.com
- NAFMNP Awarded USDA Cooperative Agreement to Continue MarketLink Program Under FFAB
- Costa Oil - 10 Minute Oil Change Surpasses 70 Locations with Construction of San Antonio, TX Stores — Eyes Growth Via Acquisition or Being Acquired
- LaTerra and Respark Under Contract with AIMCO to Acquire a $455M, 7-Property Chicago Multifamily Portfolio
This non-dilutive acquisition funding is in addition to the over $60 million in potential equity funding previously offered upon public listing of HOPE Therapeutics shares on a public exchange. Additional information to be presented by NRXP at upcoming HC Wainwright Annual Global Investment Conference in New York, September 9-11, 2024.
NRXP has formed partnerships to license clinical trial data from a French Government-funded trial and two NIH-funded trials all of which demonstrate efficacy of racemic Intravenous ketamine against depression and two of which demonstrate statistically significant benefit vs suicidality.
In March 2024 NRXP demonstrated the formulation of a pH neutral patentable form of IV ketamine that it anticipates will have widespread applicability both in treatment of depression and chronic pain.
NRXP presented final data from the recently completed phase 2b/3 trial of NRX-101 in suicidal bipolar depression4 at the American Society of Clinical Psychopharmacology's annual meeting. These data demonstrated a significantly improved safety profile versus the standard of care, as demonstrated by a clinically significant reduction in akathisia (P=0.025) and time to sustained remission from suicidality (P=0.05). Akathisia is an adverse event seen with antidepressant medications considered by many experts to be a precursor to suicide. Given the vital need for safer medications in this at-risk population, we plan to submit an NDA for Accelerated Approval to the US FDA for treatment of bipolar depression patients with suicidality or akathisia, based on these data as well as additional data from our STABIL-B5 trial.
HOPE Therapeutics, is an NXRP subsidiary that will focus on the delivery of advance psychiatric treatments, including ketamine-focused treatment for depression and suicidality. Unlike the core business of NRXP, that is focused on biotechnology Research and Development, HOPE is organized around consolidating existing best-in-class clinics into a nationwide network. This has been done previously and without much success with clinics that are not necessarily psychiatrist-led. With Hope ownership as an asset of NRXP, this will further strengthen the NRXP balance sheet and aims to further enhance shareholder value.
More on Michimich.com
- Record Revenue, Tax Tailwinds, and AI-Driven Scale: Why Off The Hook YS Inc. Is Emerging as a Standout in the $57 Billion U.S. Marine Market
- VSee Health (N A S D A Q: VSEE) Secures $6.0M At-Market Investment, Accelerates Expansion as Revenues Surge
- Children Rising Appoints Marshelle A. Wilburn as New Executive Director
- Fairmint CEO Joris Delanoue Elected General Director of the Canton Foundation
- Sleep Basil Mattress Co.'s Debuts New Home Page Showcasing Performance Sleep Solutions for Active Denver Lifestyles
NRX-101 for Treatment of Chronic Pain:
In 2023, NRXP licensed US Patent 8,653,120 for the use of DCS in chronic pain and filed a now-accepted Investigational New Drug (IND) application with the FDA to initiate commercial drug development of NRX-101 in chronic pain. Data lock has now been achieved in a 200-person randomized prospective trial funded by the US DOD.
During Q3 2023, NRXP tested NRX-101 and its components against resistant pathogens that appear on the Congressionally mandated Qualified Infectious Disease Product (QIDP) list and proved in vitro effectiveness against antibiotic-resistant E. coli, Pseudomonas, and Acinetobacter. Accordingly, NRXP was granted QIDP designation, Fast Track Designation, and Priority Review by the US FDA in January 2024.
Should NRX-101 succeed in clinical trials, NRXP will consider developing a follow-on product that is anticipated to achieve another 20 years of patent exclusivity.
NRXP recently announced data demonstrating that NRX-101 does not compromise the intestinal microbiome, unlike common antibiotics including Clindamycin and Ciprofloxacin. Should these findings be documented in human patients, NRX-101 would represent the only treatment for cUTI that does not cause C. Difficile infection.
Additionally, should NRXP or its partners succeed in serving 10% of the cUTI market, NRXP believes that the revenue from NRX-101 has the potential to be hundreds of million annually, based on 3 million cases per year in the US and potential pricing of over $3,500/course of therapy.
Media Contact
Company Name: NRx Pharmaceuticals, Inc.
Contact Person: Matthew Duffy, Chief Business Officer
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Country: United States
Website: https://www.nrxpharma.com/
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
Filed Under: Health
0 Comments
Latest on Michimich.com
- Kentucky Judges Ignore Evidence, Prolong Father's Ordeal in Baseless Case
- Contracting Resources Group Receives 2025 HIRE Vets Platinum Medallion Award from the U.S. Department of Labor
- Detroit Manufacturing Systems Completes Successful Aquisition Of Android Industries And Avancez Form
- Crunchbase Ranks Phinge Founder & CEO Robert DeMaio #1 Globally. Meet him in Las Vegas-Week of CES to Learn About Netverse, Patented App-less Platform
- Roofman USA Encourages Ann Arbor Homeowners to Plan Ahead for Roof Replacements in 2026
- Wilderness Construction Highlights Design Details That Transform Bathrooms into Spa Sanctuaries
- Japanese Martial Arts Center Helps Students Find Strength and Balance Through Structured Training
- CMR Mechanical Shares Expert Tips to Prevent Furnace Breakdowns This Winter
- Detroit Puzzle Competition Concludes Final In-Person Round for $11,239 Prize
- IODefi Introduces New Web3 Infrastructure Framework as XRP Ledger Development Gains Global Attention
- Terizza Forms Strategic Collaboration with UC San Diego to Pioneer Next-Generation Distributed AI Infrastructure
- EnergyStrat Launches Global LNG Risk Outlook 2025–2030
- Strong Revenue Gains, Accelerating Growth, Strategic Hospital Expansion & Uplisting Advancements: Cardiff Lexington Corporation (Stock Symbol: CDIX)
- Ohana Growth Partners Expands Into Wisconsin With Its First Planet Fitness Club
- Holiday Decorations Most Likely to Cause Injuries
- UK Financial Ltd Confirms Official Corporate Structure of the Maya Preferred Project and Its Dual-Class Token System
- CCHR Florida Joins Global Call to Ban Electroshock Treatment, Citing New Evidence of Widespread Patient Harm
- BoxingRx Announces Full Gym Renovation Ahead of New Ownership's One-Year Anniversary
- UK Financial Ltd Announces It's Official Corporate Headquarters In The United Kingdom
- Rigani Press Announces Breakthrough Book for Health IT and Medical Leaders to Forge the Road to Responsible AI