Trending...
- Phinge CEO Ranked #1 Globally by Crunchbase for the Last Week, Will Be in Las Vegas Jan. 4-9, the Week of CES to Discuss Netverse & IPO Coming in 2026
- VSee Health (N A S D A Q: VSEE) Secures $6.0M At-Market Investment, Accelerates Expansion as Revenues Surge
- Costa Oil - 10 Minute Oil Change Surpasses 70 Locations with Construction of San Antonio, TX Stores — Eyes Growth Via Acquisition or Being Acquired
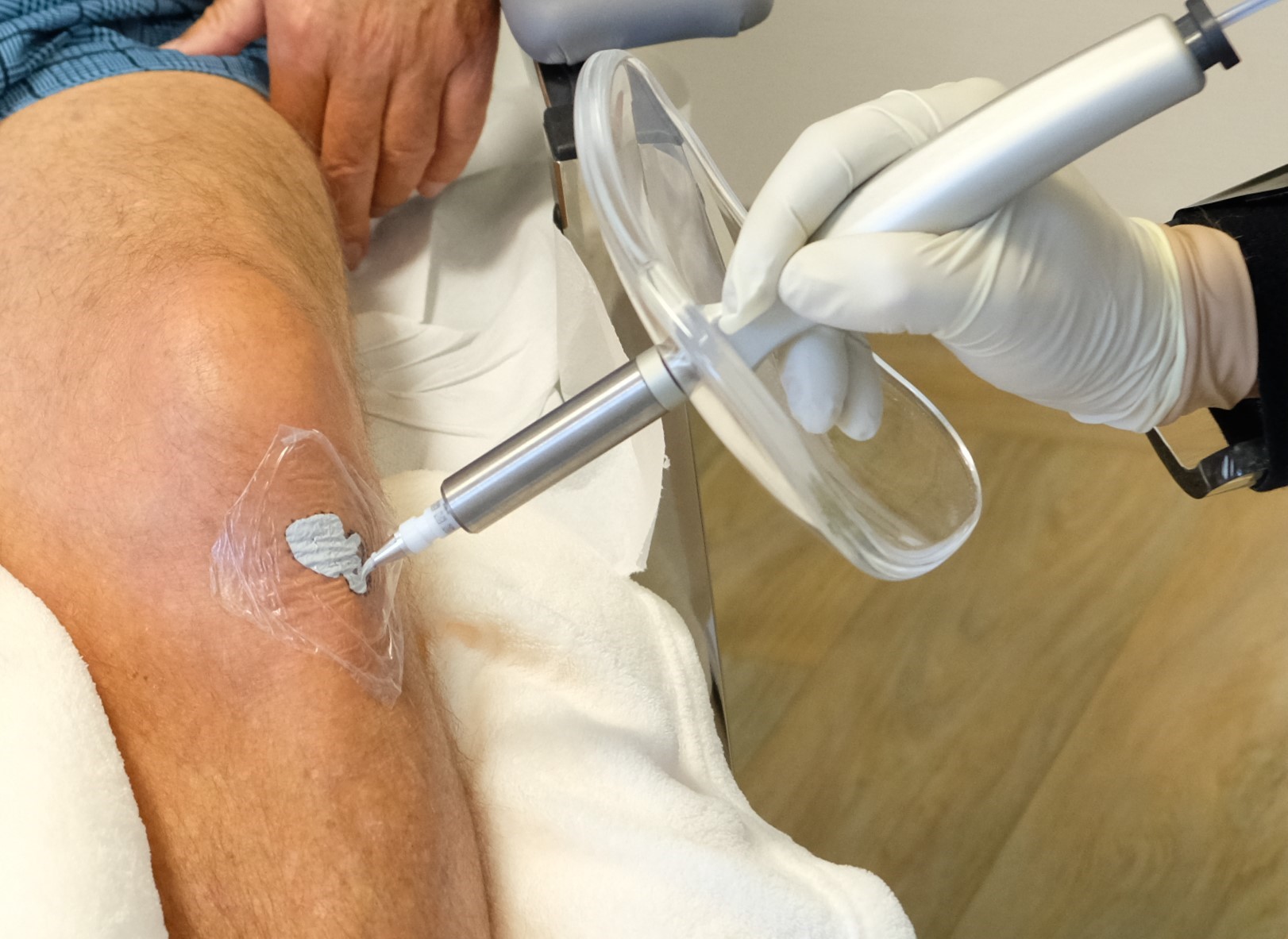
ROSTOCK, Germany - Michimich -- OncoBeta® GmbH has announced University Clinic of Rostock (is particpating in the global phase IV EPIC-Skin Study (Efficacy of Personalised Irradiation with Rhenium-SCT – for the treatment of non-melanoma skin cancer [NMSC]) treating its first patients with Rhenium-SCT® as part of the international study.
The first German patients were treated on 21st July, and are now part of 210 adults participating in the international study that will follow their progress over the next 24 months. The EPIC-Skin study is being conducted through study centres located in Australia, Austria, United Kingdom and Germany.
Rhenium-SCT® has been used in Rostock and in other parts of Germany as an NMSC treatment for some time. In addition to this EPIC-Skin Study, The University Clinic of Rostock has also been involved in a local clinical study using Rhenium-SCT, with results due to be presented later this year.
Treating physician in the EPIC-Skin study - University Clinic of Rostock, Dr. Martin Heuschkel (Nuclear Medicine) said, "Rhenium-SCT® is viewed as a favourable treatment due to its ability to be applied directly to an affected area, without harming or scarring surrounding tissue. This study presents the unqiue opportunity to further evaluate this new non-invasive epidermal radioisotope therapy and its long-term efficacy in improving patient outcomes."
There are more than 7.7 million cases of NMSC each year, and incidence rates are increasing globally1,2 with Germany currently ranked 8th in the world3. Standard treatments for NMSCs are surgery-based approaches, which may have a risk of scarring or loss of function. Rhenium-SCT® uses a non-invasive paste containing ß-emitting particles directly to the lesion, which eliminate cancer cells without the need for surgery, in one single session.4-6
Dr Gerhard Dahlhoff, Medical Director at OncoBeta GmbH, says, "The role of the EPIC-Skin Study is not to challenge the existing treatment options but rather to show Rhenium-SCT® is a treatment alternative with which NMSCs can be cured effectively, with a strong focus on patient perspectives."
The EPIC-Skin study will measure Patient Reported Outcomes such as quality of life, treatment comfort and cosmetic outcomes, as well as further evaluating the efficacy of Rhenium-SCT for the treatment of NMSC. To provide a simple and streamlined way to record their experiences, patients in the study will utilise OncoBeta's Clinical Study app.
More on Michimich.com
Shannon D. Brown III, CEO and Managing Director at OncoBeta, says, "OncoBeta's goal is to provide the best innovative solutions for patients suffering from NMSCs. Rostock is the second centre in Europe participating in the EPIC-Skin study provding important multi-centre data that will offer new insights in the treatment of NMSC's and the role of Rhenium-SCT® as a treatment alternative for patients."
Clinicians who are interested in enrolling patients in the study can contact OncoBeta directly at www.oncobeta.com/contact.
About the Rhenium-SCT® (Skin Cancer Therapy)
Non-melanoma skin cancer (NMSC) is the most common form of cancer in humans.2 The most common cause of NMSC is sun exposure, while other predisposing factors include genetic skin conditions and immunosuppressive diseases or treatments.7
The Rhenium-SCT® is a painless*, single session†, non-invasive therapy providing for unparalleled aesthetic results, even in cases otherwise considered difficult to treat.4-6 The Rhenium-SCT utilizes the radioisotope Rhenium-188 in an epidermal application with optimal properties for the treatment of NMSCs (non-melanoma skin cancers). The Rhenium-SCT is a precise, personalised therapy that is only applied to the area needed to treat without affecting the healthy tissue. The specially designed device ensures the Rhenium-SCT compound never comes in direct contact with the patient's skin and the application is safe and simple for the applying physician. Most cases of NMSCs (Basal Cell Carcinomas and Squamous Cell Carcinomas) can be treated using the Rhenium-SCT in one single session.†6 Scar-free healing of the treated lesion area and the regeneration of healthy tissue occurs usually within a few weeks after treatment.6
About OncoBeta®
OncoBeta®, with its headquarters located in Garching near Munich, Germany, is a privately held medical device company, specializing in the development and commercialization of state-of-the-art, innovative therapies. Since its foundation, OncoBeta has concentrated its efforts on the development, regulatory approval(s) and commercialization of the epidermal radioisotope therapy Rhenium-SCT® (Skin Cancer Therapy), targeting NMSCs. OncoBeta has perfected the customized application and device management system in conformity with all health, safety and environmental protection regulatory standards.
More on Michimich.com
Find out more about the Rhenium-SCT® at www.oncobeta.com
Follow us on social media:
LinkedIn: www.linkedin.com/company/oncobeta-gmbh/
Facebook: www.facebook.com/oncobeta/
Instagram: www.instagram.com/oncobeta_gmbh/
Forward-looking statements
This announcement includes forward-looking statements that involve risks, uncertainties and other factors, many of which are outside of OncoBeta's control, and which could cause actual results to differ materially from the results discussed in the forward-looking statements. Forward-looking statements include statements concerning OncoBeta's plans, objectives, goals, future events, performance and/or other information that is not historical information. All such forward-looking statements are expressly qualified by these cautionary statements and any other cautionary statements which may accompany the forward-looking statements. OncoBeta® undertakes no obligation to publicly update or revise forward-looking statements to reflect subsequent events or circumstances after the date made, except as required by law.
*No reported pain4,5
†Complete tumour regression in 98.5% of lesions treated6
References
The first German patients were treated on 21st July, and are now part of 210 adults participating in the international study that will follow their progress over the next 24 months. The EPIC-Skin study is being conducted through study centres located in Australia, Austria, United Kingdom and Germany.
Rhenium-SCT® has been used in Rostock and in other parts of Germany as an NMSC treatment for some time. In addition to this EPIC-Skin Study, The University Clinic of Rostock has also been involved in a local clinical study using Rhenium-SCT, with results due to be presented later this year.
Treating physician in the EPIC-Skin study - University Clinic of Rostock, Dr. Martin Heuschkel (Nuclear Medicine) said, "Rhenium-SCT® is viewed as a favourable treatment due to its ability to be applied directly to an affected area, without harming or scarring surrounding tissue. This study presents the unqiue opportunity to further evaluate this new non-invasive epidermal radioisotope therapy and its long-term efficacy in improving patient outcomes."
There are more than 7.7 million cases of NMSC each year, and incidence rates are increasing globally1,2 with Germany currently ranked 8th in the world3. Standard treatments for NMSCs are surgery-based approaches, which may have a risk of scarring or loss of function. Rhenium-SCT® uses a non-invasive paste containing ß-emitting particles directly to the lesion, which eliminate cancer cells without the need for surgery, in one single session.4-6
Dr Gerhard Dahlhoff, Medical Director at OncoBeta GmbH, says, "The role of the EPIC-Skin Study is not to challenge the existing treatment options but rather to show Rhenium-SCT® is a treatment alternative with which NMSCs can be cured effectively, with a strong focus on patient perspectives."
The EPIC-Skin study will measure Patient Reported Outcomes such as quality of life, treatment comfort and cosmetic outcomes, as well as further evaluating the efficacy of Rhenium-SCT for the treatment of NMSC. To provide a simple and streamlined way to record their experiences, patients in the study will utilise OncoBeta's Clinical Study app.
More on Michimich.com
- Pinealage: the app that turns strangers into meditation companions — in crowdfunding phase
- "Micro-Studio": Why San Diegans are Swapping Crowded Gyms for Private, One-on-One Training at Sweat Society
- Beycome Closes $2.5M Seed Round Led by InsurTech Fund
- Sterling Advisory Group Celebrates Two-Year Anniversary
- Tru by Hilton Columbia South Opens to Guests
Shannon D. Brown III, CEO and Managing Director at OncoBeta, says, "OncoBeta's goal is to provide the best innovative solutions for patients suffering from NMSCs. Rostock is the second centre in Europe participating in the EPIC-Skin study provding important multi-centre data that will offer new insights in the treatment of NMSC's and the role of Rhenium-SCT® as a treatment alternative for patients."
Clinicians who are interested in enrolling patients in the study can contact OncoBeta directly at www.oncobeta.com/contact.
About the Rhenium-SCT® (Skin Cancer Therapy)
Non-melanoma skin cancer (NMSC) is the most common form of cancer in humans.2 The most common cause of NMSC is sun exposure, while other predisposing factors include genetic skin conditions and immunosuppressive diseases or treatments.7
The Rhenium-SCT® is a painless*, single session†, non-invasive therapy providing for unparalleled aesthetic results, even in cases otherwise considered difficult to treat.4-6 The Rhenium-SCT utilizes the radioisotope Rhenium-188 in an epidermal application with optimal properties for the treatment of NMSCs (non-melanoma skin cancers). The Rhenium-SCT is a precise, personalised therapy that is only applied to the area needed to treat without affecting the healthy tissue. The specially designed device ensures the Rhenium-SCT compound never comes in direct contact with the patient's skin and the application is safe and simple for the applying physician. Most cases of NMSCs (Basal Cell Carcinomas and Squamous Cell Carcinomas) can be treated using the Rhenium-SCT in one single session.†6 Scar-free healing of the treated lesion area and the regeneration of healthy tissue occurs usually within a few weeks after treatment.6
About OncoBeta®
OncoBeta®, with its headquarters located in Garching near Munich, Germany, is a privately held medical device company, specializing in the development and commercialization of state-of-the-art, innovative therapies. Since its foundation, OncoBeta has concentrated its efforts on the development, regulatory approval(s) and commercialization of the epidermal radioisotope therapy Rhenium-SCT® (Skin Cancer Therapy), targeting NMSCs. OncoBeta has perfected the customized application and device management system in conformity with all health, safety and environmental protection regulatory standards.
More on Michimich.com
- Christy Sports donates $56K in new gear to SOS Outreach to help kids hit the slopes
- "BigPirate" Sets Sail: A New Narrative-Driven Social Casino Adventure
- Phinge CEO Ranked #1 Globally by Crunchbase for the Last Week, Will Be in Las Vegas Jan. 4-9, the Week of CES to Discuss Netverse & IPO Coming in 2026
- Women's Everyday Safety Is Changing - The Blue Luna Shows How
- Microgaming Unveils Red Papaya: A New Studio Delivering Cutting-Edge, Feature-Rich Slots
Find out more about the Rhenium-SCT® at www.oncobeta.com
Follow us on social media:
LinkedIn: www.linkedin.com/company/oncobeta-gmbh/
Facebook: www.facebook.com/oncobeta/
Instagram: www.instagram.com/oncobeta_gmbh/
Forward-looking statements
This announcement includes forward-looking statements that involve risks, uncertainties and other factors, many of which are outside of OncoBeta's control, and which could cause actual results to differ materially from the results discussed in the forward-looking statements. Forward-looking statements include statements concerning OncoBeta's plans, objectives, goals, future events, performance and/or other information that is not historical information. All such forward-looking statements are expressly qualified by these cautionary statements and any other cautionary statements which may accompany the forward-looking statements. OncoBeta® undertakes no obligation to publicly update or revise forward-looking statements to reflect subsequent events or circumstances after the date made, except as required by law.
*No reported pain4,5
†Complete tumour regression in 98.5% of lesions treated6
References
- Global Burden of Disease Cancer Collaboration, et al. JAMA Oncol. 2019;5(12):1749-1768.
- Ciążyńska M, et al. Sci Rep. 2021;11(1):4337.
- https://www.wcrf.org/cancer-trends/skin-cancer-statistics/
- Cipriani C, et al. J Dermatolog Treat. 2020; Jul 22:1-7.
- Sedda AF, et al. Clin Exp Dermatol. 2008;33(6):745-749.
- Cipriani C, Sedda AF. Epidermal Radionuclide Therapy - Dermatological High-Dose-Rate. Brachytherapy for the Treatment of Basal and Squamous Cell Carcinoma. In: Therapeutic Nuclear Medicine, editor Baum RP; New York: Springer, 2014.
- Cancer.net. Skin Cancer (Non-Melanoma): Risk Factors and Prevention. October 2020. https://www.cancer.net/cancer-types/skin-cancer-non-melanoma/risk-factors-and-prevention (accessed November 2021).
Source: OncoBeta GmbH
Filed Under: Health
0 Comments
Latest on Michimich.com
- LaTerra and Respark Under Contract with AIMCO to Acquire a $455M, 7-Property Chicago Multifamily Portfolio
- Record Revenue, Tax Tailwinds, and AI-Driven Scale: Why Off The Hook YS Inc. Is Emerging as a Standout in the $57 Billion U.S. Marine Market
- VSee Health (N A S D A Q: VSEE) Secures $6.0M At-Market Investment, Accelerates Expansion as Revenues Surge
- Children Rising Appoints Marshelle A. Wilburn as New Executive Director
- Fairmint CEO Joris Delanoue Elected General Director of the Canton Foundation
- Sleep Basil Mattress Co.'s Debuts New Home Page Showcasing Performance Sleep Solutions for Active Denver Lifestyles
- Bent Danholm Joins The American Dream TV as Central Florida Host
- The Nature of Miracles Celebrates 20th Anniversary Third Edition Published by DreamMakers Enterprises LLC
- IconicVan Launches Modular Aluminum Flooring and High-Capacity Shelving for Commercial Vans
- Artificial Intelligence Leader Releases Children's Book on Veterans Day
- Felicia Allen Hits #1 Posthumously with "Christmas Means Worship"
- "4 for $5" Mitten Eats recipes make healthy eating affordable
- CCHR Documentary Probes Growing Evidence Linking Psychiatric Drugs to Violence
- Tokenized Real-World Assets: Iguabit Brings Institutional Investment Opportunities to Brazil
- MEX Finance meluncurkan platform keuangan berbasis riset yang berfokus pada data, logika, dan efisiensi pengambilan keputusan investasi
- From MelaMed Wellness to Calmly Rooted: A New Chapter in Functional Wellness
- New Angles US Group Founder Alexander Harrington Receives Top U.S. Corporate Training Honor and Leads Asia-Pacific Engagements in Taiwan
- UK Financial Ltd Board of Directors Establishes Official News Distribution Framework and Issues Governance Decision on Official Telegram Channels
- UK Financial Ltd Sets Official 30-Day Conversion Deadline for Three Exchange Listed Tokens Ahead of Regulated Upgrade
- New Jersey Therapy and Life Coaching Unveils Original Dan Fenelon Mural in Voorhees New Jersey Therapy Office