Trending...
- Phinge CEO Ranked #1 Globally by Crunchbase for the Last Week, Will Be in Las Vegas Jan. 4-9, the Week of CES to Discuss Netverse & IPO Coming in 2026
- VSee Health (N A S D A Q: VSEE) Secures $6.0M At-Market Investment, Accelerates Expansion as Revenues Surge
- Costa Oil - 10 Minute Oil Change Surpasses 70 Locations with Construction of San Antonio, TX Stores — Eyes Growth Via Acquisition or Being Acquired
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) $NRXP Set Up for $300 Million in Milestones on Tiered Double-Digit Royalties
MIAMI - Michimich -- Term Sheet Signed for $2.5 Million Strategic Investment from a Global Medical Device Manufacturer into NRXP Subsidiary HOPE Therapeutics.
Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Binding Letter of Intent with Neurospa TMS Holdings, LLC for Expansion of its Planned International Network of Interventional Psychiatry Clinics.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
Poised to Address Over $3 Billion Suicidal Depression Market in the US.
Application to Uplist to NASDAQ Global Market from NASDAQ Capital Market
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year. This represents a $3-5 billion market at expected pricing. Based on the data in the trials referenced above, the Company's regulatory counsel encouraged the Company to file an NDA for suicidal depression for NRX-100.
In a January 2025 report, respected investment analysis firm D. Boral Capital assigned NRXP a $31 Price Target. The full report may be viewed at this link: https://www.nrxpharma.com/wp-content/uploads/2025/01/HOPE-Therapeutics-Advances-With-Kadima.pdf.
More on Michimich.com
Term Sheet for Strategic Investment from a Global Medical Device Manufacturer into NRXP Subsidiary HOPE Therapeutics
On April 3rd NRXP announced signing of a term sheet with a global medical device manufacturer, as anticipated in the Company's recently filed annual report. The investor shares NRXP subsidiary HOPE's vision of providing comprehensive interventional psychiatry treatments to patients around the world. This Investment is intended to support initiation of HOPE's network of clinics to treat suicidal depression and PTSD with ketamine, TMS and other modalities.
The Term Sheet, which is non-binding and subject to the execution of a definitive Stock Purchase Agreement, contemplates an investment of $2.5 million to purchase Series A Convertible Preferred Stock at a $50 million pre-money valuation. This investment, together with expected bank financing and current balance sheet assets, is anticipated to close concurrent with, and in support of, the closing of HOPE's recently announced clinic acquisitions.
Binding Letter of Intent with Neurospa TMS Holdings, LLC for Expansion of its Planned International Network of Interventional Psychiatry Clinics
On March 24th NRXP announced signing of a binding Letter of Intent to acquire a majority interest in Neurospa TMS Holdings, LLC. Neurospa operates six interventional psychiatry clinics on Florida's Gulf Coast and will constitute a key element of HOPE's Florida network going forward. Neurospa is revenue generating and EBITDA positive.
Neurospa leverages state-of-the-art interventional psychiatry procedures, including Ketamine Infusion Therapy, Transcranial Magnetic Stimulation (TMS), and Spravato®, augmented by traditional psychiatry and talk therapy to provide a full continuum of care for people with depression, suicidality, PTSD, anxiety, and related disorders. TMS is an FDA-approved procedure in which focused electromagnetic treatment has been demonstrated to reduce symptoms of depression. Ketamine and Spravato® are similarly known to reduce symptoms of depression and both forms of treatment are increasingly used in an additive manner.
Fourth Quarter and Full Year 2024 Financial Results and Provides Corporate Update
On March 17th NRXP announced its financial results for the quarter and year ended December 31, 2024, and provided a business update. The announcement included the following key highlights:
NRXP initiated filing of a New Drug Application ("NDA") to the FDA for NRX-100 (IV Ketamine) for the treatment of Suicidal Depression; planned filing of an NDA for Accelerated Approval under Breakthrough Designation and Priority Review of NRX-101 for the treatment of bipolar depression in people at risk of akathisia. Both have anticipated PDUFA dates prior to December 31, 2025
NRXP has accepted non-binding potential terms from a commercial pharmaceutical company to license and distribute NRX-100, providing over $300 million in milestones plus tiered double-digit royalties based on net sales
More on Michimich.com
NRXP retained a leading regulatory law firm to file a citizen's petition with the US Food and Drug Administration ("FDA") to remove benzethonium chloride – a toxic preservative -- from presentations of ketamine intended for intravenous use; planned 2Q25 filing of an Abbreviated New Drug Application ("ANDA") for the use of preservative-free ketamine in all current indications
Wholly owned subsidiary HOPE Therapeutics, signed non-binding letters of intent to acquire three precision psychiatry centers and is currently completing financial due diligence and definitive agreements. Currently negotiating the terms for the acquisition of six additional centers
The HOPE acquisitions are planned to form the foundation for a national network offering interventional psychiatry services to treat suicidal depression, post-traumatic stress disorder ("PTSD") and related conditions
NRXP received and negotiating a term sheet from a publicly-traded strategic investor currently engaged in manufacturing Transcranial Magnetic Stimulation ("TMS") devices to provide capital in support of expansion of further HOPE clinic acquisitions.
NRXP has engaged BTIG as financial advisor for clinic acquisition and capital formation; leading global financial services firm specializing in investment banking, institutional trading, research, and related brokerage services for strategic growth opportunities.
NRXP regained compliance with the NASDAQ market value of listed securities ("MVLS") requirement.
Substantially reduced operating costs compared to prior year
Management continues to forecast, although no assurances can be given, profitability on a forward-looking run-rate basis by year end 2025
NRXP filed Module 3 (manufacturing) of its New Drug Application ("NDA") for NRX-100 (preservative-free sterile IV ketamine) in a tamper-resistant, diversion resistant packaging presentation in the fourth quarter of 2024. NRX-100 was previously granted Fast Track Designation by FDA in combination with use of NRX-101. Ketamine efficacy data from four clinical trials are intended to support the filing. Three manufacturing lots are now complete, with filed stability data suitable for shelf life exceeding two years at room temperature. The anticipated PDUFA date for this NDA is prior to December 31, 2025.
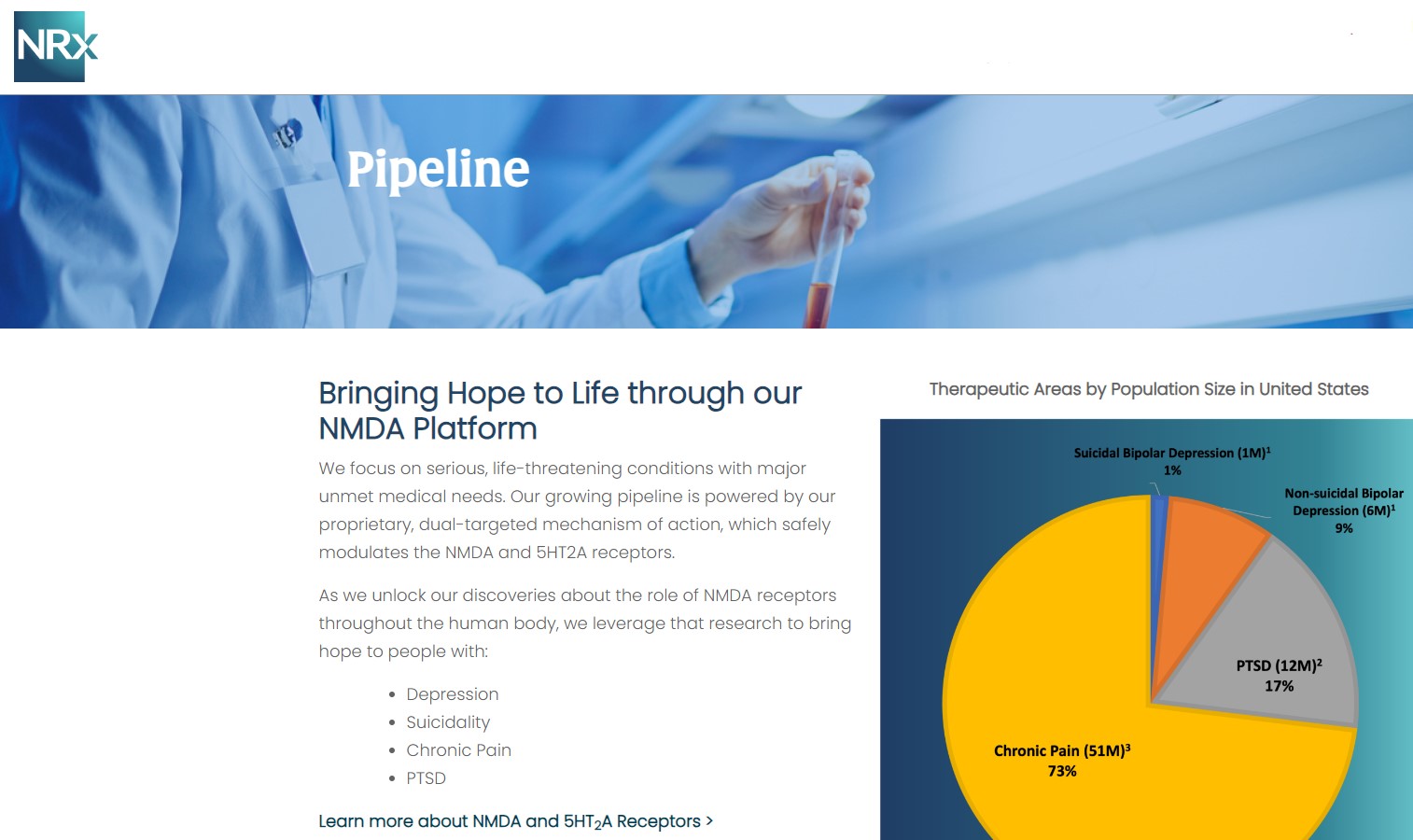
NRX-100 is poised to address the over $3 billion Suicidal Depression market in the US.
NRXP has accepted non-binding potential terms from a commercial pharmaceutical company to license and distribute NRX-100 (preservative-free IV ketamine) that provides for over $300 million in potential milestones plus a tiered double-digit royalty, subject to further due diligence and finalized agreement.
NRXP estimates that the market for the initial indication is over $2 billion, while the broad bipolar market could exceed $5 billion.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Binding Letter of Intent with Neurospa TMS Holdings, LLC for Expansion of its Planned International Network of Interventional Psychiatry Clinics.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
Poised to Address Over $3 Billion Suicidal Depression Market in the US.
Application to Uplist to NASDAQ Global Market from NASDAQ Capital Market
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year. This represents a $3-5 billion market at expected pricing. Based on the data in the trials referenced above, the Company's regulatory counsel encouraged the Company to file an NDA for suicidal depression for NRX-100.
In a January 2025 report, respected investment analysis firm D. Boral Capital assigned NRXP a $31 Price Target. The full report may be viewed at this link: https://www.nrxpharma.com/wp-content/uploads/2025/01/HOPE-Therapeutics-Advances-With-Kadima.pdf.
More on Michimich.com
- Pinealage: the app that turns strangers into meditation companions — in crowdfunding phase
- "Micro-Studio": Why San Diegans are Swapping Crowded Gyms for Private, One-on-One Training at Sweat Society
- Beycome Closes $2.5M Seed Round Led by InsurTech Fund
- Sterling Advisory Group Celebrates Two-Year Anniversary
- Tru by Hilton Columbia South Opens to Guests
Term Sheet for Strategic Investment from a Global Medical Device Manufacturer into NRXP Subsidiary HOPE Therapeutics
On April 3rd NRXP announced signing of a term sheet with a global medical device manufacturer, as anticipated in the Company's recently filed annual report. The investor shares NRXP subsidiary HOPE's vision of providing comprehensive interventional psychiatry treatments to patients around the world. This Investment is intended to support initiation of HOPE's network of clinics to treat suicidal depression and PTSD with ketamine, TMS and other modalities.
The Term Sheet, which is non-binding and subject to the execution of a definitive Stock Purchase Agreement, contemplates an investment of $2.5 million to purchase Series A Convertible Preferred Stock at a $50 million pre-money valuation. This investment, together with expected bank financing and current balance sheet assets, is anticipated to close concurrent with, and in support of, the closing of HOPE's recently announced clinic acquisitions.
Binding Letter of Intent with Neurospa TMS Holdings, LLC for Expansion of its Planned International Network of Interventional Psychiatry Clinics
On March 24th NRXP announced signing of a binding Letter of Intent to acquire a majority interest in Neurospa TMS Holdings, LLC. Neurospa operates six interventional psychiatry clinics on Florida's Gulf Coast and will constitute a key element of HOPE's Florida network going forward. Neurospa is revenue generating and EBITDA positive.
Neurospa leverages state-of-the-art interventional psychiatry procedures, including Ketamine Infusion Therapy, Transcranial Magnetic Stimulation (TMS), and Spravato®, augmented by traditional psychiatry and talk therapy to provide a full continuum of care for people with depression, suicidality, PTSD, anxiety, and related disorders. TMS is an FDA-approved procedure in which focused electromagnetic treatment has been demonstrated to reduce symptoms of depression. Ketamine and Spravato® are similarly known to reduce symptoms of depression and both forms of treatment are increasingly used in an additive manner.
Fourth Quarter and Full Year 2024 Financial Results and Provides Corporate Update
On March 17th NRXP announced its financial results for the quarter and year ended December 31, 2024, and provided a business update. The announcement included the following key highlights:
NRXP initiated filing of a New Drug Application ("NDA") to the FDA for NRX-100 (IV Ketamine) for the treatment of Suicidal Depression; planned filing of an NDA for Accelerated Approval under Breakthrough Designation and Priority Review of NRX-101 for the treatment of bipolar depression in people at risk of akathisia. Both have anticipated PDUFA dates prior to December 31, 2025
NRXP has accepted non-binding potential terms from a commercial pharmaceutical company to license and distribute NRX-100, providing over $300 million in milestones plus tiered double-digit royalties based on net sales
More on Michimich.com
- Christy Sports donates $56K in new gear to SOS Outreach to help kids hit the slopes
- "BigPirate" Sets Sail: A New Narrative-Driven Social Casino Adventure
- Phinge CEO Ranked #1 Globally by Crunchbase for the Last Week, Will Be in Las Vegas Jan. 4-9, the Week of CES to Discuss Netverse & IPO Coming in 2026
- Women's Everyday Safety Is Changing - The Blue Luna Shows How
- Microgaming Unveils Red Papaya: A New Studio Delivering Cutting-Edge, Feature-Rich Slots
NRXP retained a leading regulatory law firm to file a citizen's petition with the US Food and Drug Administration ("FDA") to remove benzethonium chloride – a toxic preservative -- from presentations of ketamine intended for intravenous use; planned 2Q25 filing of an Abbreviated New Drug Application ("ANDA") for the use of preservative-free ketamine in all current indications
Wholly owned subsidiary HOPE Therapeutics, signed non-binding letters of intent to acquire three precision psychiatry centers and is currently completing financial due diligence and definitive agreements. Currently negotiating the terms for the acquisition of six additional centers
The HOPE acquisitions are planned to form the foundation for a national network offering interventional psychiatry services to treat suicidal depression, post-traumatic stress disorder ("PTSD") and related conditions
NRXP received and negotiating a term sheet from a publicly-traded strategic investor currently engaged in manufacturing Transcranial Magnetic Stimulation ("TMS") devices to provide capital in support of expansion of further HOPE clinic acquisitions.
NRXP has engaged BTIG as financial advisor for clinic acquisition and capital formation; leading global financial services firm specializing in investment banking, institutional trading, research, and related brokerage services for strategic growth opportunities.
NRXP regained compliance with the NASDAQ market value of listed securities ("MVLS") requirement.
Substantially reduced operating costs compared to prior year
Management continues to forecast, although no assurances can be given, profitability on a forward-looking run-rate basis by year end 2025
NRXP filed Module 3 (manufacturing) of its New Drug Application ("NDA") for NRX-100 (preservative-free sterile IV ketamine) in a tamper-resistant, diversion resistant packaging presentation in the fourth quarter of 2024. NRX-100 was previously granted Fast Track Designation by FDA in combination with use of NRX-101. Ketamine efficacy data from four clinical trials are intended to support the filing. Three manufacturing lots are now complete, with filed stability data suitable for shelf life exceeding two years at room temperature. The anticipated PDUFA date for this NDA is prior to December 31, 2025.
NRX-100 is poised to address the over $3 billion Suicidal Depression market in the US.
NRXP has accepted non-binding potential terms from a commercial pharmaceutical company to license and distribute NRX-100 (preservative-free IV ketamine) that provides for over $300 million in potential milestones plus a tiered double-digit royalty, subject to further due diligence and finalized agreement.
NRXP estimates that the market for the initial indication is over $2 billion, while the broad bipolar market could exceed $5 billion.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
Filed Under: Health
0 Comments
Latest on Michimich.com
- LaTerra and Respark Under Contract with AIMCO to Acquire a $455M, 7-Property Chicago Multifamily Portfolio
- Record Revenue, Tax Tailwinds, and AI-Driven Scale: Why Off The Hook YS Inc. Is Emerging as a Standout in the $57 Billion U.S. Marine Market
- VSee Health (N A S D A Q: VSEE) Secures $6.0M At-Market Investment, Accelerates Expansion as Revenues Surge
- Children Rising Appoints Marshelle A. Wilburn as New Executive Director
- Fairmint CEO Joris Delanoue Elected General Director of the Canton Foundation
- Sleep Basil Mattress Co.'s Debuts New Home Page Showcasing Performance Sleep Solutions for Active Denver Lifestyles
- Bent Danholm Joins The American Dream TV as Central Florida Host
- The Nature of Miracles Celebrates 20th Anniversary Third Edition Published by DreamMakers Enterprises LLC
- IconicVan Launches Modular Aluminum Flooring and High-Capacity Shelving for Commercial Vans
- Artificial Intelligence Leader Releases Children's Book on Veterans Day
- Felicia Allen Hits #1 Posthumously with "Christmas Means Worship"
- "4 for $5" Mitten Eats recipes make healthy eating affordable
- CCHR Documentary Probes Growing Evidence Linking Psychiatric Drugs to Violence
- Tokenized Real-World Assets: Iguabit Brings Institutional Investment Opportunities to Brazil
- MEX Finance meluncurkan platform keuangan berbasis riset yang berfokus pada data, logika, dan efisiensi pengambilan keputusan investasi
- From MelaMed Wellness to Calmly Rooted: A New Chapter in Functional Wellness
- New Angles US Group Founder Alexander Harrington Receives Top U.S. Corporate Training Honor and Leads Asia-Pacific Engagements in Taiwan
- UK Financial Ltd Board of Directors Establishes Official News Distribution Framework and Issues Governance Decision on Official Telegram Channels
- UK Financial Ltd Sets Official 30-Day Conversion Deadline for Three Exchange Listed Tokens Ahead of Regulated Upgrade
- New Jersey Therapy and Life Coaching Unveils Original Dan Fenelon Mural in Voorhees New Jersey Therapy Office