Trending...
- Entering 2026 with Expanding Footprint, Strong Industry Tailwinds, and Anticipated Q3 Results: Off The Hook YS Inc. (N Y S E American: OTH)
- Crunchbase Ranks Phinge Founder & CEO Robert DeMaio #1 Globally. Meet him in Las Vegas-Week of CES to Learn About Netverse, Patented App-less Platform
- Contracting Resources Group Receives 2025 HIRE Vets Platinum Medallion Award from the U.S. Department of Labor
BOZEN, Italy - Michimich -- OncoBeta®, a leading medical device company specialising in innovative epidermal radioisotope therapies, has announced the expansion of Rhenium-SCT® (Skin Cancer Therapy) treatment services in Italy with the Bolzano Hospital the latest to come on board. This marks a significant advancement in the availability of non-melanoma skin cancer (NMSC) treatment options within the country.
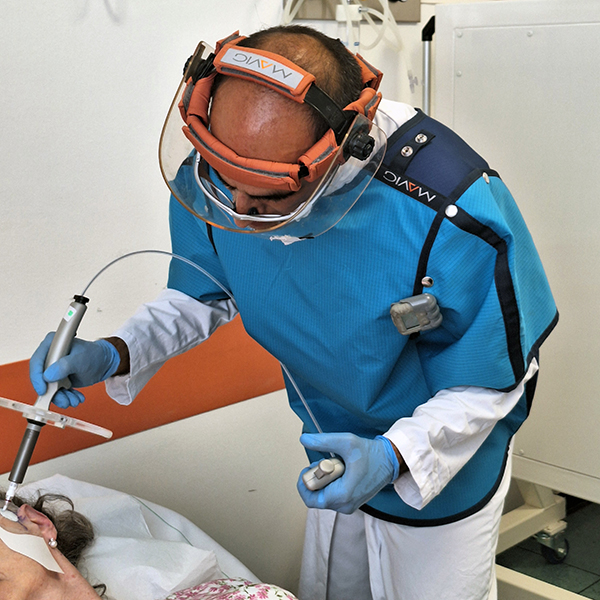
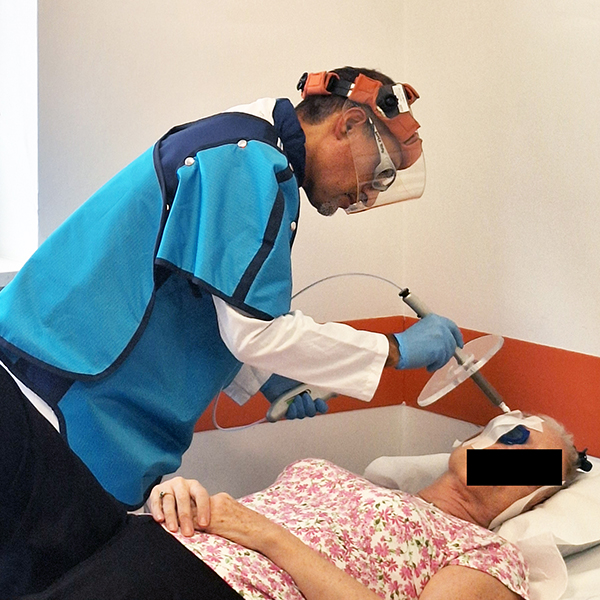
Rhenium skin cancer therapy is an advanced radionuclide therapy that offers a painless,*1,2 single-session,†1-3 non-invasive‡1 treatment without disfiguring scarring.1,4 Treatment with Rhenium-SCT employs a superficial application of a paste containing β-emitting particles directly over the NMSC lesion, targeting cancer cells without the need for surgery.1,4
Dr. Mohsen Farsad at Bolzano Hospital has been impressed with the Rhenium-SCT device, noting, "I have witnessed impressive outcomes with several patients treated here in Italy already, and am pleased with the design of the device technology."
"Rhenium skin cancer therapy fulfills many patients' desires for a non-surgical alternative for the treatment of NMSCs especially in relation to difficult localisations. I am pleased to continue treating suitable patients with Rhenium-SCT", adds Dr. Farsad.
Shannon D. Brown III, CCO at OncoBeta, says, "While surgical intervention remains the standard-of-care for NMSC treatment, it often leads to suboptimal outcomes. Rhenium-SCT offers a non-surgical alternative with a simple application directly to the area needed to treat, and allows patients to resume their daily activities with minimal disruption."
According to GLOBOCAN estimates, the number of new NMSC cases in Italy is projected to rise from 29,000 to 41,700 between 2022 and 2045.5 Epidemiological data for Europe shows an annual incidence rate of NMSCs at 129.3 cases per 100,000 men and 90.8 per 100,000 women. Basal cell carcinoma (BCC) accounts for approximately 15% of all diagnosed cancers in Italy, with an annual incidence of around 100 cases per 100,000 inhabitants.6
Dr. Gerhard Dahlhoff, Medical Director at OncoBeta, emphasised, "Rhenium-SCT enables targeted, non-invasive treatment of NMSCs while preserving adjacent healthy tissue. This patient-friendly approach underscores our commitment to enhancing treatment options for those affected by NMSCs."
More on Michimich.com
Rhenium-SCT is currently available in Germany, Austria, Switzerland, Italy, Spain, United Kingdom, South Africa and Australia. Rhenium-SCT will be provided to patients at Bolzano Hospital by OncoBeta's distribution partner, DSD Pharma.
About the Rhenium-SCT® (Skin Cancer Therapy)
Non-melanoma skin cancer (NMSC) is the most common form of cancer in humans.1 The most common cause of NMSC is sun exposure, while other predisposing factors include genetic skin conditions and immunosuppressive diseases or treatments.1,4
The Rhenium-SCT is a painless,*1,2 single session,†1-3 non-invasive‡1 therapy that provides aesthetic results, even in cases otherwise considered difficult to treat.1,4,7 The Rhenium-SCT utilizes the radioisotope rhenium-188 in an epidermal application with optimal properties for the treatment of NMSCs (non-melanoma skin cancers). The Rhenium-SCT is a precise, personalized therapy that is only applied to the area needed to treat without affecting the healthy tissue.4,7 The specially designed device ensures the Rhenium-SCT compound never comes in direct contact with the patient's skin and the application is safe and simple for the applying physician. Most cases of NMSCs (basal cell carcinomas and squamous cell carcinomas) can be treated using the Rhenium-SCT in one single session. †1-4 Scar-free healing of the treated lesion area and the regeneration of healthy tissue occurs usually within a few weeks after treatment.1,7,8
About OncoBeta®
OncoBeta® with its headquarters located in Garching near Munich, Germany, is a privately held medical device company, specializing in the development and commercialization of state-of-the-art, innovative therapies. Since its foundation, OncoBeta has concentrated its efforts on the development, regulatory approval(s) and commercialization of the epidermal radioisotope therapy Rhenium-SCT® (Skin Cancer Therapy), targeting NMSCs. OncoBeta has perfected the customized application and device management system in conformity with all health, safety, and environmental protection regulatory standards.
Find out more about the Rhenium-SCT at www.oncobeta.com
Follow us on social media:
More on Michimich.com
LinkedIn: https://www.linkedin.com/company/oncobeta-gmbh/
Facebook: https://www.facebook.com/oncobeta/
Instagram: https://www.instagram.com/oncobeta_gmbh/
About DSD Pharma
DSD Pharma is a distributor for diagnostics, theranostics and radiopharmaceutical/medical devices in the field of nuclear medicine and radiopharmaceuticals. DSD Pharma offers a product portfolio developed together at the forefront of research to its customers. This ensures the safety of latest technologies for the HCP and the best available diagnosis/theranosis/therapy for the patient.
Find out more about DSD Pharma at www.dsd-pharma.com
Forward-looking statements
This announcement includes forward-looking statements that involve risks, uncertainties and other factors, many of which are outside of OncoBeta's control and which could cause actual results to differ materially from the results discussed in the forward-looking statements. Forward-looking statements include statements concerning OncoBeta's plans, objectives, goals, future events, performance and/or other information that is not historical information. All such forward-looking statements are expressly qualified by these cautionary statements and any other cautionary statements which may accompany the forward-looking statements. OncoBeta undertakes no obligation to publicly update or revise forward-looking statements to reflect subsequent events or circumstances after the date made, except as required by law.
*No reported pain during procedure.1,2
†Complete tumour regression in 98.5% of lesions treated.3
‡A treatment is considered non-invasive when no cut or break in the skin is created.9
References
Rhenium skin cancer therapy is an advanced radionuclide therapy that offers a painless,*1,2 single-session,†1-3 non-invasive‡1 treatment without disfiguring scarring.1,4 Treatment with Rhenium-SCT employs a superficial application of a paste containing β-emitting particles directly over the NMSC lesion, targeting cancer cells without the need for surgery.1,4
Dr. Mohsen Farsad at Bolzano Hospital has been impressed with the Rhenium-SCT device, noting, "I have witnessed impressive outcomes with several patients treated here in Italy already, and am pleased with the design of the device technology."
"Rhenium skin cancer therapy fulfills many patients' desires for a non-surgical alternative for the treatment of NMSCs especially in relation to difficult localisations. I am pleased to continue treating suitable patients with Rhenium-SCT", adds Dr. Farsad.
Shannon D. Brown III, CCO at OncoBeta, says, "While surgical intervention remains the standard-of-care for NMSC treatment, it often leads to suboptimal outcomes. Rhenium-SCT offers a non-surgical alternative with a simple application directly to the area needed to treat, and allows patients to resume their daily activities with minimal disruption."
According to GLOBOCAN estimates, the number of new NMSC cases in Italy is projected to rise from 29,000 to 41,700 between 2022 and 2045.5 Epidemiological data for Europe shows an annual incidence rate of NMSCs at 129.3 cases per 100,000 men and 90.8 per 100,000 women. Basal cell carcinoma (BCC) accounts for approximately 15% of all diagnosed cancers in Italy, with an annual incidence of around 100 cases per 100,000 inhabitants.6
Dr. Gerhard Dahlhoff, Medical Director at OncoBeta, emphasised, "Rhenium-SCT enables targeted, non-invasive treatment of NMSCs while preserving adjacent healthy tissue. This patient-friendly approach underscores our commitment to enhancing treatment options for those affected by NMSCs."
More on Michimich.com
- Fairmint CEO Joris Delanoue Elected General Director of the Canton Foundation
- Sleep Basil Mattress Co.'s Debuts New Home Page Showcasing Performance Sleep Solutions for Active Denver Lifestyles
- Bent Danholm Joins The American Dream TV as Central Florida Host
- The Nature of Miracles Celebrates 20th Anniversary Third Edition Published by DreamMakers Enterprises LLC
- IconicVan Launches Modular Aluminum Flooring and High-Capacity Shelving for Commercial Vans
Rhenium-SCT is currently available in Germany, Austria, Switzerland, Italy, Spain, United Kingdom, South Africa and Australia. Rhenium-SCT will be provided to patients at Bolzano Hospital by OncoBeta's distribution partner, DSD Pharma.
About the Rhenium-SCT® (Skin Cancer Therapy)
Non-melanoma skin cancer (NMSC) is the most common form of cancer in humans.1 The most common cause of NMSC is sun exposure, while other predisposing factors include genetic skin conditions and immunosuppressive diseases or treatments.1,4
The Rhenium-SCT is a painless,*1,2 single session,†1-3 non-invasive‡1 therapy that provides aesthetic results, even in cases otherwise considered difficult to treat.1,4,7 The Rhenium-SCT utilizes the radioisotope rhenium-188 in an epidermal application with optimal properties for the treatment of NMSCs (non-melanoma skin cancers). The Rhenium-SCT is a precise, personalized therapy that is only applied to the area needed to treat without affecting the healthy tissue.4,7 The specially designed device ensures the Rhenium-SCT compound never comes in direct contact with the patient's skin and the application is safe and simple for the applying physician. Most cases of NMSCs (basal cell carcinomas and squamous cell carcinomas) can be treated using the Rhenium-SCT in one single session. †1-4 Scar-free healing of the treated lesion area and the regeneration of healthy tissue occurs usually within a few weeks after treatment.1,7,8
About OncoBeta®
OncoBeta® with its headquarters located in Garching near Munich, Germany, is a privately held medical device company, specializing in the development and commercialization of state-of-the-art, innovative therapies. Since its foundation, OncoBeta has concentrated its efforts on the development, regulatory approval(s) and commercialization of the epidermal radioisotope therapy Rhenium-SCT® (Skin Cancer Therapy), targeting NMSCs. OncoBeta has perfected the customized application and device management system in conformity with all health, safety, and environmental protection regulatory standards.
Find out more about the Rhenium-SCT at www.oncobeta.com
Follow us on social media:
More on Michimich.com
- Artificial Intelligence Leader Releases Children's Book on Veterans Day
- Felicia Allen Hits #1 Posthumously with "Christmas Means Worship"
- "4 for $5" Mitten Eats recipes make healthy eating affordable
- CCHR Documentary Probes Growing Evidence Linking Psychiatric Drugs to Violence
- Tokenized Real-World Assets: Iguabit Brings Institutional Investment Opportunities to Brazil
LinkedIn: https://www.linkedin.com/company/oncobeta-gmbh/
Facebook: https://www.facebook.com/oncobeta/
Instagram: https://www.instagram.com/oncobeta_gmbh/
About DSD Pharma
DSD Pharma is a distributor for diagnostics, theranostics and radiopharmaceutical/medical devices in the field of nuclear medicine and radiopharmaceuticals. DSD Pharma offers a product portfolio developed together at the forefront of research to its customers. This ensures the safety of latest technologies for the HCP and the best available diagnosis/theranosis/therapy for the patient.
Find out more about DSD Pharma at www.dsd-pharma.com
Forward-looking statements
This announcement includes forward-looking statements that involve risks, uncertainties and other factors, many of which are outside of OncoBeta's control and which could cause actual results to differ materially from the results discussed in the forward-looking statements. Forward-looking statements include statements concerning OncoBeta's plans, objectives, goals, future events, performance and/or other information that is not historical information. All such forward-looking statements are expressly qualified by these cautionary statements and any other cautionary statements which may accompany the forward-looking statements. OncoBeta undertakes no obligation to publicly update or revise forward-looking statements to reflect subsequent events or circumstances after the date made, except as required by law.
*No reported pain during procedure.1,2
†Complete tumour regression in 98.5% of lesions treated.3
‡A treatment is considered non-invasive when no cut or break in the skin is created.9
References
- Castellucci P, et al. Eur J Nucl Med Mol Imaging. 2021; 48: 1511–1521.
- Cipriani C, et al. J Dermatol Treat. 2022;33(2):969–975. Epub 22 Jul 2020.
- Cipriani C, et al. In Therapeutic Nuclear Medicine. 2014. RP Baum (Ed), New York: Springer.
- Tietze JK, et al. Clin Nucl Med. 2023;48:869–76.
- Global Burden of Disease Cancer Collaboration, et al. JAMA Oncol. 2019;5(12):1749-1768.
- Sordi E, et al. Epidemiologia. 2024;5:1–10.
- Cipriani C, et al. International J Nucl Med. 2017; July:114–112.
- Sedda AF, et al. Clin Exp Dermatol. 2008;33(6):745–749.
- Australian Therapeutic Goods Administration. Acronym and glossary of terms. Available at: https://www.tga.gov.au/resources/acronyms-and-glossary-terms. (Accessed June 2024).
Source: OncoBeta GmbH
Filed Under: Health
0 Comments
Latest on Michimich.com
- Detroit Puzzle Competition Concludes Final In-Person Round for $11,239 Prize
- IODefi Introduces New Web3 Infrastructure Framework as XRP Ledger Development Gains Global Attention
- Terizza Forms Strategic Collaboration with UC San Diego to Pioneer Next-Generation Distributed AI Infrastructure
- EnergyStrat Launches Global LNG Risk Outlook 2025–2030
- Strong Revenue Gains, Accelerating Growth, Strategic Hospital Expansion & Uplisting Advancements: Cardiff Lexington Corporation (Stock Symbol: CDIX)
- Ohana Growth Partners Expands Into Wisconsin With Its First Planet Fitness Club
- Holiday Decorations Most Likely to Cause Injuries
- UK Financial Ltd Confirms Official Corporate Structure of the Maya Preferred Project and Its Dual-Class Token System
- CCHR Florida Joins Global Call to Ban Electroshock Treatment, Citing New Evidence of Widespread Patient Harm
- BoxingRx Announces Full Gym Renovation Ahead of New Ownership's One-Year Anniversary
- UK Financial Ltd Announces It's Official Corporate Headquarters In The United Kingdom
- Rigani Press Announces Breakthrough Book for Health IT and Medical Leaders to Forge the Road to Responsible AI
- FreeTo.Chat - The bold, Anonymous Confession Platform, ushers in a new era of tension relief
- Female Founders Institute Announces Special Performance by Caitlin McGrath at the 2026 Charity Gala
- Hyatt House Fresno Celebrates Grand Opening, Introducing the First Hyatt House in Fresno, California
- "I Make Music Not Excuses" Journal by Anthony Clint Jr. Becomes International Amazon Best Seller, Empowering Music Creators Worldwide
- Novi Glass Company Says Why Custom Mirrors are Better
- Michigan Debt Collection Agency Helps Creditors Start the New Year Fresh!
- Ann Arbor Chiropractor Uses ESWT to Treat Elbow Pain
- Ann Arbor Computer Service Shares the Spirit of Giving!