Trending...
- Inside the Fight for Affordable Housing: Avery Headley Joins Terran Lamp for a Candid Bronx Leadership Conversation
- UK Financial Ltd Announces A Special Board Meeting Today At 4PM: Orders MCAT Lock on CATEX, Adopts ERC-3643 Standard, & Cancels $0.20 MCOIN for $1
- Verb™ Presents Features Vanguard Personalized Indexing: Utilizing Advanced Tax-Loss Harvesting Technology
$NRXP Has Manufactured Multiple Commercial Lots of NRX-100 and KETAFREE™ with a Shelf Life of Three Years.
MIAMI - Michimich -- NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is rapidly emerging as one of the most compelling growth stories in mental-health therapeutics. With three revenue-generating treatment facilities now operating in Florida—and six expected by year-end—the company is entering 2026 with accelerating clinical operations, expanding market share, and advancing two FDA-directed regulatory pathways for ketamine-based therapeutics aimed at suicidal depression, one of the largest unmet needs in mental health.
Backed by strong clinical data, FDA Fast Track designations, and third-party revenue projections, NRXP appears positioned to capitalize on a rapidly expanding U.S. ketamine market currently estimated at $750 million, projected to reach $3.35 billion globally by 2034.
Adding to investor enthusiasm, analyst D. Boral issued a Buy rating and a $34 price target, citing NRXP's accelerating execution and diversified commercialization strategy.
A Mission Targeting One of America's Most Pressing Health Crises
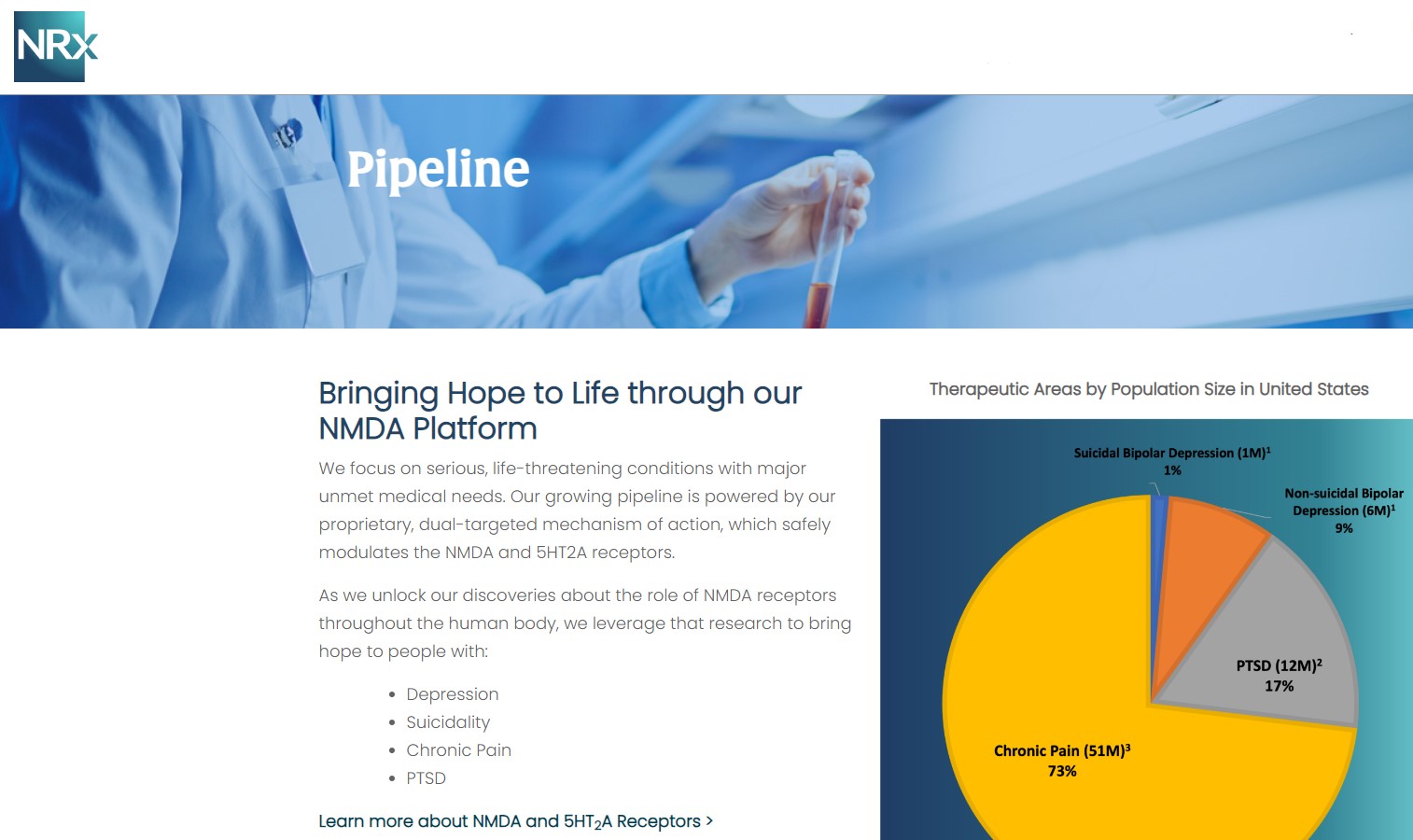
More than 13 million Americans seriously consider suicide each year (CDC). NRXP is developing next-generation therapeutics aimed directly at this crisis through its NMDA-based drug platform:
Key Pipeline Assets
NRXP is partnered with Alvogen Pharmaceuticals for the development and commercialization of NRX-101.
More on Michimich.com
A Dual-Path Strategy for NRX-100: Innovative and Generic Market Entry
NRXP's regulatory strategy is unique: it is simultaneously pursuing both an innovative NDA pathway and a generic ANDA pathway for NRX-100 and KETAFREE™.
This creates two powerful value drivers:
1. Innovative NDA for Suicidal Depression
2. ANDA for KETAFREE™ (Generic Ketamine)
With commercial lots already manufactured, NRXP is positioned for a swift commercial rollout upon approval.
HOPE Clinics: Expanding Revenue Footprint Across Florida
NRXP's HOPE Therapeutics subsidiary is advancing a scalable clinic model for ketamine and advanced TMS-based interventions.
Current & Expected Expansion
These facilities support:
Management expects increased revenue contributions from clinical operations through 2026.
Breakthrough ONE-D: First-in-Florida Deployment with Ampa Health
In November, NRXP launched patient treatment using Ampa Health's ONE-D protocol—a groundbreaking approach reporting:
ONE-D combines:
ONE-D is being deployed at HOPE clinics in Sarasota, Naples, and Fort Myers, with additional locations coming online in 2025.
More on Michimich.com
This marks a major competitive differentiator for NRXP in the mental-health treatment sector.
Financial Position: Capital Secured Through July 2026
NRXP reported securing sufficient operating capital to support its drug development programs through July 2026, complementing increasing clinical revenues.
Third-quarter results (released November 17, 2025) highlighted:
The full Q3 update and webcast are available on the NRXP investor relations site.
High-Value Licensing Opportunity
NRXP has accepted non-binding potential terms from an international pharmaceutical partner for the licensing and distribution of NRX-100. Terms include:
If finalized, this could represent a transformative revenue opportunity for the company.
Investment Outlook
With:
NRx Pharmaceuticals is attracting increased attention from both institutional and retail investors.
The recent $34 price target from D. Boral underscores growing confidence in NRXP's ability to execute on a scalable, high-margin strategy targeting one of the most underserved and urgent areas of mental health.
For More Information
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Website: www.nrxpharma.com
Chief Business Officer: Matthew Duffy
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Backed by strong clinical data, FDA Fast Track designations, and third-party revenue projections, NRXP appears positioned to capitalize on a rapidly expanding U.S. ketamine market currently estimated at $750 million, projected to reach $3.35 billion globally by 2034.
Adding to investor enthusiasm, analyst D. Boral issued a Buy rating and a $34 price target, citing NRXP's accelerating execution and diversified commercialization strategy.
A Mission Targeting One of America's Most Pressing Health Crises
More than 13 million Americans seriously consider suicide each year (CDC). NRXP is developing next-generation therapeutics aimed directly at this crisis through its NMDA-based drug platform:
Key Pipeline Assets
- NRX-100 (IV Ketamine):
- FDA Fast Track designation for suicidal ideation in depression, including bipolar depression.
- Multiple commercial lots produced with three-year shelf life stability.
- NDA submission expected in Q4 2025, supported by real-world patient data from more than 60,000 IV ketamine cases.
- KETAFREE™ (preservative-free ketamine):
- Pursuing approval via an ANDA (generic) pathway.
- Recently received supportive FDA correspondence confirming no major deficiencies and on track for a Q2 2026 GDUFA date.
- Addresses a ketamine market worth ~$750 million annually.
- NRX-101 (D-cycloserine + lurasidone):
- FDA-designated Investigational Breakthrough Therapy.
- Targets suicidal treatment-resistant bipolar depression, chronic pain, and potential utility in complicated UTIs.
- New real-world data suggest its active ingredient doubles the effectiveness of TMS—a potential major indication expansion.
NRXP is partnered with Alvogen Pharmaceuticals for the development and commercialization of NRX-101.
More on Michimich.com
- Tiger-Rock Martial Arts Appoints Jami Bond as Vice President of Growth
- Super League (N A S D A Q: SLE) Enters Breakout Phase: New Partnerships, Zero Debt & $20 Million Growth Capital Position Company for 2026 Acceleration
- Finland's Gambling Reform Promises "Single-Click" Block for All Licensed Sites
- Private Keys Are a Single Point of Failure: Security Advisor Gideon Cohen Warns MPC Technology Is Now the Only Defense for Institutional Custody
- Compliance Is the Ticket to Entry: Legal Advisor Gabriela Moraes Analyzes RWA Securitization Paths Under Brazil's New Legislation
A Dual-Path Strategy for NRX-100: Innovative and Generic Market Entry
NRXP's regulatory strategy is unique: it is simultaneously pursuing both an innovative NDA pathway and a generic ANDA pathway for NRX-100 and KETAFREE™.
This creates two powerful value drivers:
1. Innovative NDA for Suicidal Depression
- Expected completion: Q4 2025.
- Includes comparative real-world data showing IV ketamine may have faster onset and greater effect than intranasal S-ketamine.
- NRXP has applied for a Commissioner's National Priority Voucher, which could accelerate FDA review.
2. ANDA for KETAFREE™ (Generic Ketamine)
- Re-filed after FDA approved NRXP's Suitability Petition for its preservative-free formulation.
- Supportive FDA feedback in November 2025 indicated no significant deficiencies.
- A Citizen Petition filed to remove toxic preservative benzethonium chloride from ketamine products could reshape the competitive landscape.
With commercial lots already manufactured, NRXP is positioned for a swift commercial rollout upon approval.
HOPE Clinics: Expanding Revenue Footprint Across Florida
NRXP's HOPE Therapeutics subsidiary is advancing a scalable clinic model for ketamine and advanced TMS-based interventions.
Current & Expected Expansion
- 3 operational facilities in Florida
- 6 additional facilities planned by year-end
These facilities support:
- NRX-100 Expanded Access Program
- Ketamine-based clinical services
- ONE-D single-day TMS depression treatments
- Veterans, first responders, and active-duty military populations
Management expects increased revenue contributions from clinical operations through 2026.
Breakthrough ONE-D: First-in-Florida Deployment with Ampa Health
In November, NRXP launched patient treatment using Ampa Health's ONE-D protocol—a groundbreaking approach reporting:
- 87% response rate
- 72% remission
- Achieved in one day, instead of traditional 90-day TMS schedules
ONE-D combines:
- Ampa's FDA-cleared TMS device
- D-cycloserine (NRX-101 active ingredient)
- Lisdexamfetamine
ONE-D is being deployed at HOPE clinics in Sarasota, Naples, and Fort Myers, with additional locations coming online in 2025.
More on Michimich.com
- Coalition and CCHR Call on FDA to Review Electroshock Device and Consider a Ban
- Spark Announces 2025 Design Award Winners
- NEW Luxury Single-Family Homes Coming Soon to Manalapan - Pre-Qualify Today for Priority Appointments
- Dominic Pace Returns to the NCIS Franchise With Guest Role on NCIS: Origins
- Anderson Periodontal Wellness Attends 5th Joint Congress for Ceramic Implantology
This marks a major competitive differentiator for NRXP in the mental-health treatment sector.
Financial Position: Capital Secured Through July 2026
NRXP reported securing sufficient operating capital to support its drug development programs through July 2026, complementing increasing clinical revenues.
Third-quarter results (released November 17, 2025) highlighted:
- Progress across all clinical and regulatory objectives
- Expanded Fast Track designation for NRX-100
- EU and NIH data enhancing regulatory strategy
- Growth of the HOPE facility platform
- Enhanced IP and formulation positioning
The full Q3 update and webcast are available on the NRXP investor relations site.
High-Value Licensing Opportunity
NRXP has accepted non-binding potential terms from an international pharmaceutical partner for the licensing and distribution of NRX-100. Terms include:
- Over $300 million in milestone payments
- Tiered double-digit royalties
If finalized, this could represent a transformative revenue opportunity for the company.
Investment Outlook
With:
- Multiple clinical catalysts approaching
- Two regulatory pathways advancing for NRX-100
- FDA Breakthrough Therapy designations
- National leadership in cutting-edge TMS + D-cycloserine therapy
- Increasing real-world data support
- A growing network of revenue-producing treatment centers
- Newly manufactured commercial drug supply
- And a strong capital runway
NRx Pharmaceuticals is attracting increased attention from both institutional and retail investors.
The recent $34 price target from D. Boral underscores growing confidence in NRXP's ability to execute on a scalable, high-margin strategy targeting one of the most underserved and urgent areas of mental health.
For More Information
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Website: www.nrxpharma.com
Chief Business Officer: Matthew Duffy
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
Filed Under: Health
0 Comments
Latest on Michimich.com
- 6 Holiday Looks That Scream "Old Money" But Cost Less Than Your Christmas Tree
- Compressed Gas Technologies Highlights the Long-Term Value of On-Site Nitrogen Generation
- Xmas Delights Brings Show-Stopping Holiday Light Displays to Ann Arbor
- From Cheer to Courtroom: The Hidden Legal Risks in Your Holiday Eggnog
- Future of Work Training Institute Launches the #1 Global AI & Career Transformation Community
- Controversial Vegan Turns Rapper Launches First Song, "Psychopathic Tendencies."
- Inside the Fight for Affordable Housing: Avery Headley Joins Terran Lamp for a Candid Bronx Leadership Conversation
- Canterbury Hotel Group Announces the Opening of the TownePlace Suites by Marriott Portland Airport
- Trenton Launches Noel Nights: A New Holiday Tradition for Families
- Heritage at South Brunswick's Resort-Style Amenities for Any Age and Every Lifestyle
- Prudential Alarm Strengthens BOMA Support; Celebrates Team Member Finalist
- T-TECH Partners with Japan USA Precision Tools for 2026 US Market Development of the New T-TECH 5-Axis QUICK MILL™
- CIMdata Unveils its 2026 PLM Market & Industry Forum Theme
- Record Revenues, Debt-Free Momentum & Shareholder Dividend Ignite Investor Attention Ahead of 2026–2027 Growth Targets: IQSTEL (N A S D A Q: IQST)
- Midwest Enviro Solutions Helps Homeowners Avoid Mold After a Burst Pipe
- How Michigan Computer Supplies Helps Create Hybrid-Ready Tech Spaces
- Delta Industrial Showcases the Benefit of All-In-One Steel Fabrication and Concrete Construction
- New YouTube Channel Pair Launches to Bring Entertainment Nostalgia Back to Life
- BRAG Hosts Holiday Benefit — Awards 10 Student Scholarships & Honors Timberland with the Corporate Impact Award
- FittingPros Launches Industry's First Data-Driven Golf Club Fitting Directory