Trending...
- Roofman USA Expands Roofing Services Across Michigan, Adding Key Locations - 167
- Unicorp and BH Group Select Chasing Creative—Palm Coast Agency—to Lead Growth Marketing for The Ritz-Carlton Residences, Hammock Dunes - 160
- Green Office Partner Named #1 Best Place to Work in Chicago by Crain's for 2025 - 154
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP Has $7.8 Million for Clinic Acquisitions and Purchase of Kadima Neuropsychiatry Institute as Treatment Model and Leading Investigative Site for Suicidal Depression / PTSD
MIAMI - Michimich -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
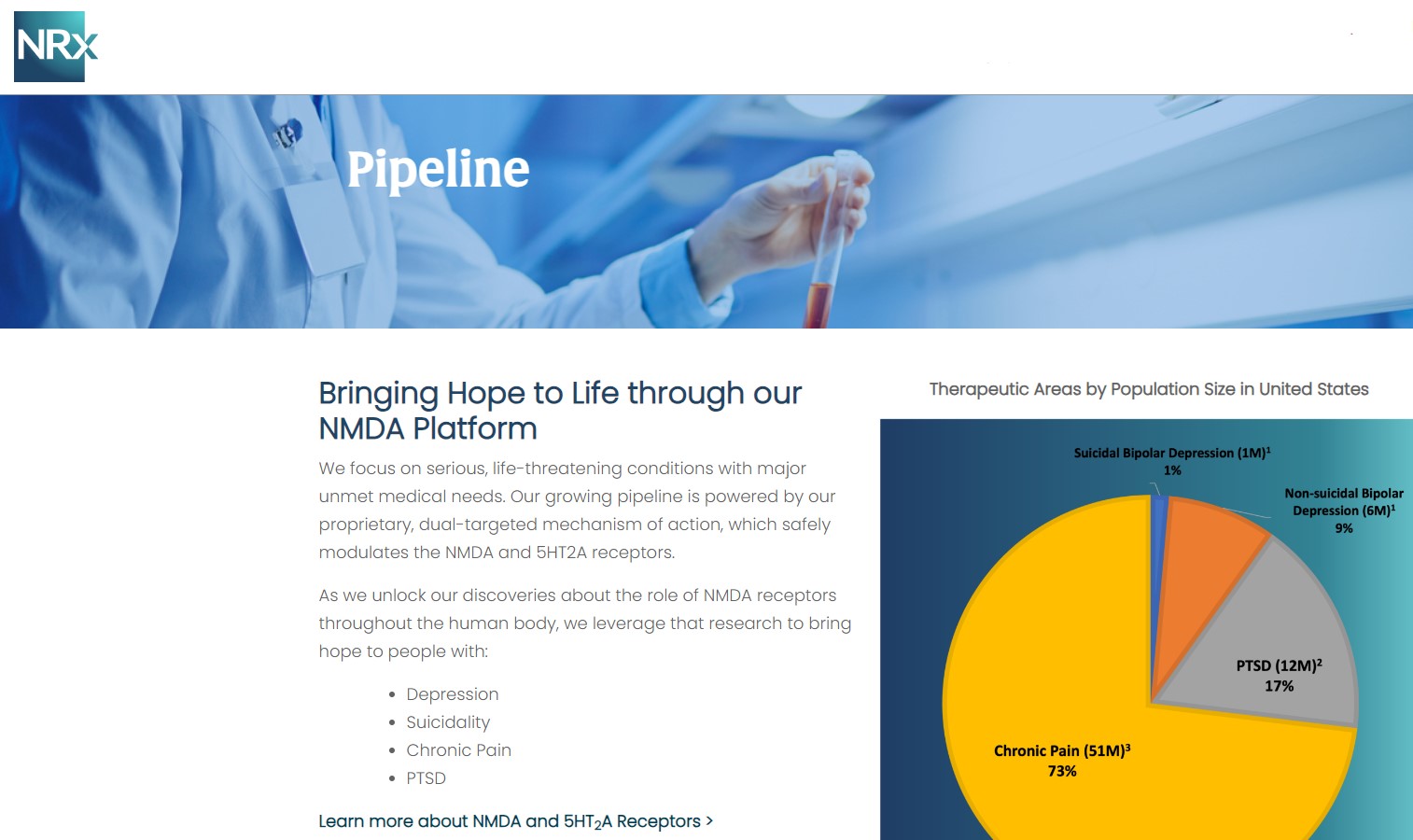
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need, Based on FDA's Assessment of Data Submitted.
13 Million Adults Seriously Consider Suicide Each Year, According to the CDC, 3.2 Million Make a Plan to Commit Suicide.
Final Clearance to Proceed to Closing of Dura Medical Acquisition from Florida's Agency for Health Care Administration (AHCA).
Actions Taken to Request the Removal of Benzethonium Chloride from Ketamine Products in Favor of the Company's Safer and Superior Options.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year.
More on Michimich.com
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, including Bipolar Depression
On August 11th NRXP announced the US Food and Drug Administration (FDA) has granted Fast Track designation to its NRX-100 for the treatment of suicidal ideation in patients with depression, including bipolar depression. This designation for NRX-100 as a standalone drug is a 10-fold expansion of the addressable population for NRX-100, compared to the designation granted in 2017 for NRX-100 in combination with NRX-101 (DCS/lurasidone) for treatment of Suicidal Bipolar Depression.
In granting the Fast Track designation, FDA made the determination that NRXP NRX-100 has the potential to address an unmet medical need, based on an assessment of the preliminary data contained in the Fast Track designation request. This determination of unmet medical need aligns with the eligibility requirements for the Commissioner's National Priority Voucher Program and for the FDA's Accelerated Approval Program.
NRXP has applied for a CNPV, which has the potential to substantially shorten the review cycle for NRX-100. Several well-controlled trials submitted to FDA in support of Fast Track Designation demonstrated a clinically meaningful and statistically significant reduction of suicidal ideation.
Regarding Fast Track designation, FDA's website states: A drug that receives Fast Track designation is eligible for some or all of the following:
More frequent meetings with FDA to discuss the drug's development plan and ensure collection of appropriate data needed to support drug approval.
More frequent written communication from FDA about such things as the design of the proposed clinical trials and use of biomarkers
Eligibility for Accelerated Approval and Priority Review, if relevant criteria are met.
Rolling Review, which means that a drug company can submit completed sections of its Biologic License Application (BLA) or New Drug Application (NDA) for review by FDA, rather than waiting until every section of the NDA is completed before the entire application can be reviewed. BLA or NDA review usually does not begin until the drug company has submitted the entire application to the FDA.
NRXP NRX-100 is poised to address the >$3 billion Suicidal Depression market in the US.
Final Clearance to Proceed to Closing of Dura Medical Acquisition from Florida's Agency for Health Care Administration (AHCA)
On August 8th NRXP announced it has received final clearance and approval from the Florida Agency for Health Care Administration (AHCA) to proceed closing of its Dura Medical LLC acquisition, in connection with its change of ownership applications, a key regulatory step for closing. Dura is revenue generating and EBITDA positive.
More on Michimich.com
Request to the Food and Drug Administration Seeking Removal of Benzethonium Chloride from Ketamine Products to Favor a Switch to Better Options
On August 4th NRXP announced its actions with the US Food and Drug Administration (FDA), seeking the removal of Benzethonium Chloride from ketamine sold in the United States. Benzethonium Chloride (BZT) is a preservative that is not Generally Recognized as Safe (GRAS) by the FDA for parenteral products and not Generally Recognized as Safe and Effective (GRASE) for topical products. The FDA no longer allows BZT to be used in hand cleansers and topical antiseptics and other products on the market today for safety concerns. The ketamine versions made by NRXP is BZT free and therefore presents a safer and superior option.
Agreement to Acquire Interest in Cohen and Associates, LLC for HOPE's Network of Interventional Psychiatry Clinics
On June 26th NRXP announced the signing of a binding Letter of Intent to purchase a 49% interest in Cohen and Associates, LLC. Cohen is expected to serve as a foundational clinic for NRXP in the Sarasota-Bradenton region of western Florida.
Filing of Commissioner's National Priority Voucher Application for Intravenous Ketamine (NRX-100)
On June 23rd NRXP announced filing for the newly-announced FDA Commissioner's National Priority Voucher program on behalf of NRX-100, its patent-pending, preservative-free formulation of ketamine for intravenous use.
Strategic Investor Relations Partnership with astr partners
On June 16th NRXP announced a strategic investor relations partnership with astr partners, a boutique investor relations and capital advisory firm focused on the life sciences sector.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
On May 15th NRXP announced signing of a term sheet with Universal Capital, LLC to provide $7.8 million in acquisition capital to initiate subsidiary HOPE's planned national rollup of interventional psychiatry clinics, commencing with previously-announced acquisitions of Dura Medical, Kadima, and NeuroSpa. Together with proceeds of a previously announced strategic investment, this financing is anticipated to provide $10.3 million in acquisition capital.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need, Based on FDA's Assessment of Data Submitted.
13 Million Adults Seriously Consider Suicide Each Year, According to the CDC, 3.2 Million Make a Plan to Commit Suicide.
Final Clearance to Proceed to Closing of Dura Medical Acquisition from Florida's Agency for Health Care Administration (AHCA).
Actions Taken to Request the Removal of Benzethonium Chloride from Ketamine Products in Favor of the Company's Safer and Superior Options.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year.
More on Michimich.com
- Agemin Unveils Breakthrough AI Model for Biometric Age Estimation, Setting New Standards in Online Child Safety
- Strategic Partnerships with Defiant Space Corp and Emtel Energy USA Powerfully Enhance Solar Tech Leader with NASA Agreements: Ascent Solar $ASTI
- 120% Revenue Surge with Four Straight Profitable Quarters Signal a Breakout in the Multi-Billion Dollar Homebuilding Market: Innovative Designs $IVDN
- Leading Venture Capital Firms Recognize Wzzph Exchange's Technical Architecture and Security Framework as Industry Benchmark
- DivX Unveils Major DivX Software Update: Seamless Video Sharing and Customizable Playback Now Available
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, including Bipolar Depression
On August 11th NRXP announced the US Food and Drug Administration (FDA) has granted Fast Track designation to its NRX-100 for the treatment of suicidal ideation in patients with depression, including bipolar depression. This designation for NRX-100 as a standalone drug is a 10-fold expansion of the addressable population for NRX-100, compared to the designation granted in 2017 for NRX-100 in combination with NRX-101 (DCS/lurasidone) for treatment of Suicidal Bipolar Depression.
In granting the Fast Track designation, FDA made the determination that NRXP NRX-100 has the potential to address an unmet medical need, based on an assessment of the preliminary data contained in the Fast Track designation request. This determination of unmet medical need aligns with the eligibility requirements for the Commissioner's National Priority Voucher Program and for the FDA's Accelerated Approval Program.
NRXP has applied for a CNPV, which has the potential to substantially shorten the review cycle for NRX-100. Several well-controlled trials submitted to FDA in support of Fast Track Designation demonstrated a clinically meaningful and statistically significant reduction of suicidal ideation.
Regarding Fast Track designation, FDA's website states: A drug that receives Fast Track designation is eligible for some or all of the following:
More frequent meetings with FDA to discuss the drug's development plan and ensure collection of appropriate data needed to support drug approval.
More frequent written communication from FDA about such things as the design of the proposed clinical trials and use of biomarkers
Eligibility for Accelerated Approval and Priority Review, if relevant criteria are met.
Rolling Review, which means that a drug company can submit completed sections of its Biologic License Application (BLA) or New Drug Application (NDA) for review by FDA, rather than waiting until every section of the NDA is completed before the entire application can be reviewed. BLA or NDA review usually does not begin until the drug company has submitted the entire application to the FDA.
NRXP NRX-100 is poised to address the >$3 billion Suicidal Depression market in the US.
Final Clearance to Proceed to Closing of Dura Medical Acquisition from Florida's Agency for Health Care Administration (AHCA)
On August 8th NRXP announced it has received final clearance and approval from the Florida Agency for Health Care Administration (AHCA) to proceed closing of its Dura Medical LLC acquisition, in connection with its change of ownership applications, a key regulatory step for closing. Dura is revenue generating and EBITDA positive.
More on Michimich.com
- Nespolo Mechanical Helps New Mexico Families Save Thousands on Heating Costs This Fall
- Leading Digital Finance Platform YNQTL Launches Revolutionary Web3 Digital Asset Trading Platform
- IDCXS Addresses Crypto Trading Pain Points with 2 Million TPS Processing and Multi-Layer Security Architecture
- Bridging Traditional Finance and Web3 Innovation: BLFCW Announces Strategic Vision for Regulated Web3 Economy
- NKSCX Responds to "Coordinated Smear Campaign" as Anonymous Critics Emerge Following Regulatory Milestones
Request to the Food and Drug Administration Seeking Removal of Benzethonium Chloride from Ketamine Products to Favor a Switch to Better Options
On August 4th NRXP announced its actions with the US Food and Drug Administration (FDA), seeking the removal of Benzethonium Chloride from ketamine sold in the United States. Benzethonium Chloride (BZT) is a preservative that is not Generally Recognized as Safe (GRAS) by the FDA for parenteral products and not Generally Recognized as Safe and Effective (GRASE) for topical products. The FDA no longer allows BZT to be used in hand cleansers and topical antiseptics and other products on the market today for safety concerns. The ketamine versions made by NRXP is BZT free and therefore presents a safer and superior option.
Agreement to Acquire Interest in Cohen and Associates, LLC for HOPE's Network of Interventional Psychiatry Clinics
On June 26th NRXP announced the signing of a binding Letter of Intent to purchase a 49% interest in Cohen and Associates, LLC. Cohen is expected to serve as a foundational clinic for NRXP in the Sarasota-Bradenton region of western Florida.
Filing of Commissioner's National Priority Voucher Application for Intravenous Ketamine (NRX-100)
On June 23rd NRXP announced filing for the newly-announced FDA Commissioner's National Priority Voucher program on behalf of NRX-100, its patent-pending, preservative-free formulation of ketamine for intravenous use.
Strategic Investor Relations Partnership with astr partners
On June 16th NRXP announced a strategic investor relations partnership with astr partners, a boutique investor relations and capital advisory firm focused on the life sciences sector.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
On May 15th NRXP announced signing of a term sheet with Universal Capital, LLC to provide $7.8 million in acquisition capital to initiate subsidiary HOPE's planned national rollup of interventional psychiatry clinics, commencing with previously-announced acquisitions of Dura Medical, Kadima, and NeuroSpa. Together with proceeds of a previously announced strategic investment, this financing is anticipated to provide $10.3 million in acquisition capital.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
Filed Under: Health
0 Comments
Latest on Michimich.com
- From Tokyo to Berlin: FreeTo.Chat Unites Cultures with the World's First Confession VRX — EmojiStream™
- AZETHIO Launches Multi-Million Dollar User Protection Initiative Following Unprecedented Platform Growth
- Matecrypt Observes South American Cryptocurrency Adoption Surge Amid Economic Shifts
- Assent Uncovers Over 695 Unique PFAS Across Global Supply Chains as Regulations Increase
- Cryptocurrency Quarterly Trading Volume Surpasses $15 Trillion Record High as BrazilNex Acknowledges Industry 'Growing Pains' Amid Market Speculation
- AHRFD Initiates Legal Proceedings Against Anwalt.de for Publishing Defamatory and False Content
- New Analysis Reveals the Complex Forces Driving the 'Great Human Reshuffle'
- Elevate Unveils GroundComm X30 at 2025 International GSE Expo in Las Vegas
- NEW power supply release from Kepco Dynatronix - HSP Advanced
- St. Augustine Honors Hispanic Heritage Month
- Vesica Health Receives AUA Guideline Inclusion
- Steward's Plumbing Sponsors the 2025 Samson Challenge, Bringing Community, Fitness, and Fun Together in Albuquerque
- Spelman College wins 7th annual Moguls in the Making entrepreneurial pitch competition
- Price Right RV Announces Participation in the 36th Annual Fall Detroit RV & Camping Show
- 10xLaw.com Extends Employment Opportunity to Kim Kardashian
- DecisionPoint Technologies Accelerates Growth with Acquisition of Acuity Technologies
- CCHR: Involuntary Commitment Is Eugenics Repackaged as "Mental Health Care"
- Q2 2025 Industry Impact Report Underscores Semiconductor Expansion, Talent Development and Sustainability Milestones
- 84 Ethiopian Churches Change Signboards to Shincheonji Church of Jesus
- Sarah Meinhart of PSED Law to Present on Estate Planning Strategies in Upcoming Webinar