Trending...
- Kaltra Launches Next-Gen MCHEdesign With Full Integration Into MCHEselect — Instant Simulation & Seamless Microchannel Coil Workflow - 171
- A Well-Fed World, Youth Climate Save and PAN International Launch PHRESH: A Global Directory of Plant-Based Hunger Relief Organizations - 157
- Nextvisit Co-Founder Ryan Yannelli Identifies Six Critical Factors for Behavioral Health Providers Evaluating AI Scribes in 2026 - 114
FAYETTEVILLE, Ark. - Michimich -- Lineus Medical is pleased to announce the appointment of Matt Stuckert to its Board of Directors. Stuckert was previously a member of the Lineus Technical Advisory Board. Stuckert brings over a decade of sales leadership experience in the vascular access space, and has held key roles at Magnolia Medical, Becton Dickinson, CareFusion, Cardinal Health, Enturia, and Venetec.. Throughout his career, he has successfully built and led sales teams, and has driven commercial growth, particularly in the adoption of novel new vascular technologies.
His expertise in sales strategy and business development will be instrumental in guiding Lineus Medical's continued success. With a deep understanding of the vascular access market, Stuckert's knowledge will help support the growth of the company's flagship product, SafeBreak Vascular. Stuckert commented, "I am excited to join Lineus Medical's Board of Directors and contribute to the company's growth. SafeBreak Vascular is a game-changing vascular access device that directly addresses IV complications and improves patient experience, both critical issues in hospitals. I look forward to leveraging my experience to help expand SafeBreak's adoption and support the company's mission of improving IV care."
More on Michimich.com
Vance Clement, CEO of Lineus Medical said, "Matt Stuckert's extensive experience in vascular access sales and marketing makes him a valuable addition to our Board. His insights will be instrumental as we expand SafeBreak Vascular's presence in hospitals nationwide. We are excited to have him join our board as we continue to revolutionize what vascular access care looks like."
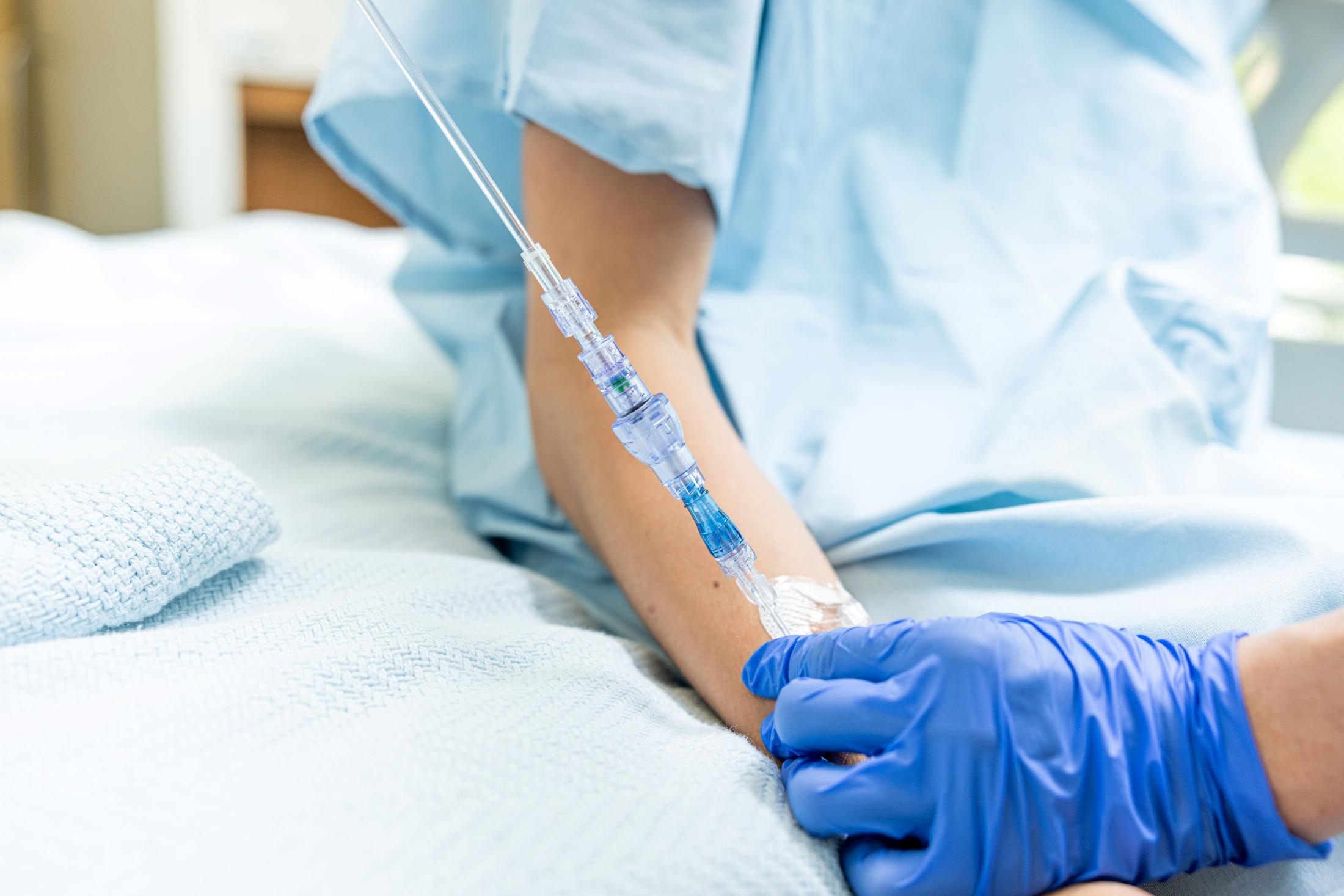
SafeBreak Vascular is the only break-away device for IV lines clinically proven to reduce IV complications¹. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close, preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patient's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money.1
About Lineus Medical
Lineus Medical is the developer of break-away technology proven to reduce IV restarts and complications¹. Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn.
References:
1. Data on File
His expertise in sales strategy and business development will be instrumental in guiding Lineus Medical's continued success. With a deep understanding of the vascular access market, Stuckert's knowledge will help support the growth of the company's flagship product, SafeBreak Vascular. Stuckert commented, "I am excited to join Lineus Medical's Board of Directors and contribute to the company's growth. SafeBreak Vascular is a game-changing vascular access device that directly addresses IV complications and improves patient experience, both critical issues in hospitals. I look forward to leveraging my experience to help expand SafeBreak's adoption and support the company's mission of improving IV care."
More on Michimich.com
- TheOneLofi2: New Home for Chill Lo-Fi Hip Hop Beats Launches on YouTube
- eJoule Inc Participates in Silicon Dragon CES 2026
- HBZBZL Unveils "Intelligent Ecosystem" Strategy: Integrating AI Analytics with Web3 Incubation
- Kaltra Launches Next-Gen MCHEdesign With Full Integration Into MCHEselect — Instant Simulation & Seamless Microchannel Coil Workflow
- A Well-Fed World, Youth Climate Save and PAN International Launch PHRESH: A Global Directory of Plant-Based Hunger Relief Organizations
Vance Clement, CEO of Lineus Medical said, "Matt Stuckert's extensive experience in vascular access sales and marketing makes him a valuable addition to our Board. His insights will be instrumental as we expand SafeBreak Vascular's presence in hospitals nationwide. We are excited to have him join our board as we continue to revolutionize what vascular access care looks like."
SafeBreak Vascular is the only break-away device for IV lines clinically proven to reduce IV complications¹. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close, preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patient's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money.1
About Lineus Medical
Lineus Medical is the developer of break-away technology proven to reduce IV restarts and complications¹. Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn.
References:
1. Data on File
Source: Lineus Medical
0 Comments
Latest on Michimich.com
- Documentary "Prescription for Violence: Psychiatry's Deadly Side Effects" Premieres, Exposes Link Between Psychiatric Drugs and Acts of Mass Violence
- Price Improvement on Luxurious Lāna'i Townhome with Stunning Ocean Views
- Nextvisit Co-Founder Ryan Yannelli Identifies Six Critical Factors for Behavioral Health Providers Evaluating AI Scribes in 2026
- CredHub and Real Property Management Join Forces to Empower Franchise Owners with Rental Payment Credit Reporting Solutions
- Leimert Park Announces Weeklong Kwanzaa Festival & Kwanzaa Parade Celebrating Black History, Culture, and Community
- Renowned Alternative Medicine Specialist Dr. Sebi and His African Bio Mineral Balance Therapy Are the Focus of New Book
- Psychiatric Drug Damage Ignored for Decades; CCHR Demands Federal Action
- Why Millions Are Losing Sexual Sensation, And Why It's Not Age, Hormones, or Desire
- Justin Jeansonne An Emerging Country Singer-Songwriter Music Fans Have Been Waiting For…a True Maverick
- Russellville Huntington Learning Center Expands Access to Literacy Support; Approved Provider Under Arkansas Department of Education
- UK Financial Ltd Launches U.S. Operations Following Delaware Approval
- Boondocking Magazine Expands FREE Digital Access for Off-Grid Camping Community
- Sterling Heights Resident Survey Shows Strong Approval of Quality of Life and City Services
- Sterling Heights Introduces First-Ever Little Free Sled Library at Delia Park
- Sterling Heights: Beginner 2-5-3.0 Level
- Sterling Heights: Dodge Park Ice Rink Opening Day
- Planet Fitness Partners With Toys For Tots To Bring Holiday Joy To Livingston County Families With F
- Pinealage: the app that turns strangers into meditation companions — in crowdfunding phase
- "Micro-Studio": Why San Diegans are Swapping Crowded Gyms for Private, One-on-One Training at Sweat Society
- Beycome Closes $2.5M Seed Round Led by InsurTech Fund