Trending...
- $5 - $20 Million in Sales for 2026; $25 - $40 Million for 2027 Projected with NASA Agreements; New MOU Signed to Improve Solar Tech in Space - 179
- Shincheonji Tanzania Church Holds Revelation Bible Exam with Local Pastors and Believers - 122
- Growth Acceleration via Strategic Reverse Split After $10 Million Acquisition for Concerts.com and TicketStub.com; AI Powered Sports/Entertainment Co - 118
MUNICH - Michimich -- OncoBeta® GmbH, a medical device company specialising in innovative epidermal radioisotope therapies, has announced 12-months interim results from its international Phase IV, multi-centre clinical trial evaluating the efficacy and safety of Rhenium-SCT® (Skin Cancer Therapy) in patients with non-melanoma skin cancer (NMSC).
The EPIC-Skin Study (Efficacy of Personalised Irradiation with Rhenium-SCT for the Treatment of Non-Melanoma Skin Cancer) is designed to assess treatment efficacy and safety, as well as key patient-reported outcomes including quality of life, treatment comfort, and cosmetic results.1 The study is based on the clinically validated effect of the beta-emitter rhenium-188 in treating basal cell carcinoma (BCC) and squamous cell carcinoma (SCC).2
Treatment data is available for 184 adult patients (with 254 lesions) across 7 study centres in Australia, Austria, Germany, the United Kingdom, and South Africa. All participants had histologically confirmed stage I or II NMSC. The median patient age was 70.3 years (range 27–95 years), and 53.3% of participants were male. Patients had one (71.2%), two (19.6%), or three (9.2%) lesions treated, which were located on the head & neck (11.8%), trunk (11.8%), lower limbs (11.8%), or upper limbs (9.1%).1
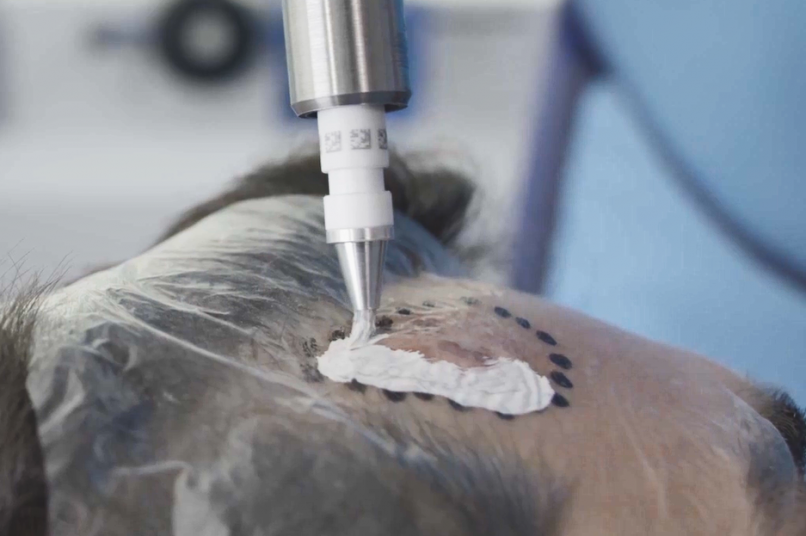
Treatment & Methodology
Rhenium-SCT was administered as a single 50-Gy topical dose of rhenium-188 embedded in a resin applied via adhesive film to the lesion site. Efficacy was assessed using modified RECIST criteria, at 12 months follow-up. Quality of life was assessed using the Skin Cancer Index (SCI) at baseline, 6 months, and 12 months. Treatment comfort was assessed using a patient questionnaire, whilst patient- and clinician-evaluated cosmetic outcomes were determined using a visual analogue scale (VAS) grading of 0-10 (0 = very poor; 10 = no wound detectable). Safety was assessed via treatment-emergent adverse events (TEAEs) and CTCAE (Common Terminology Criteria for Adverse Events) grading.
Key 12-Month Results:1
These findings confirm that Rhenium-SCT®, administered in a single session, delivers sustained high efficacy and improved quality of life, with excellent cosmetic outcomes and minimal adverse effects.
More on Michimich.com
"Rhenium-SCT® has consistently demonstrated effectiveness and safety in prior studies," said Dr. Gerhard Dahlhoff, Medical Director at OncoBeta®. "This 12-month interim data further reinforces its value as a non-invasive, targeted treatment option for NMSC, particularly for patients seeking alternatives to surgery due to cosmetic or health-related concerns."
"The 12-month results from the EPIC-Skin Study represent a major milestone for OncoBeta®," added Shannon D. Brown III, CCO OncoBeta & Managing Director Europe. "These results support the efficacy, safety, and patient satisfaction associated with Rhenium-SCT®. These are the three foundational pillars of our patient-first vision, emphasising not only clinical outcomes, but also the patient experience in achieving them."
_
About the Rhenium-SCT® (Skin Cancer Therapy)
Non-melanoma skin cancer (NMSC) is the most common form of cancer in humans.3 The most common cause of NMSC is sun exposure, while other predisposing factors include genetic skin conditions and immunosuppressive diseases or treatments.4
The Rhenium-SCT is a painless*, non-invasive‡ therapy that provides aesthetic results, even in cases otherwise considered difficult to treat.1,5,6 The Rhenium-SCT utilises the radioisotope Rhenium-188 in an epidermal application with optimal properties for the treatment of NMSCs (non-melanoma skin cancers). The Rhenium-SCT is a precise, personalised therapy2 that is only applied to the area needed to treat without affecting the healthy tissue. The specially designed device ensures the Rhenium-SCT compound never comes in direct contact with the patient's skin and the application is safe and simple for the applying physician. Scar-free healing of the treated lesion area and the regeneration of healthy tissue occurs usually within a few weeks after treatment.6-8
About OncoBeta®
OncoBeta® with its headquarters located in Garching near Munich, Germany, is a privately held medical device company, specializing in the development and commercialization of state-of-the-art, innovative therapies. Since its foundation, OncoBeta® has concentrated its efforts on the development, regulatory approval(s) and commercialization of the epidermal radioisotope therapy Rhenium-SCT® (Skin Cancer Therapy), targeting non-melanoma skin cancers and keloid scars. OncoBeta® has perfected the customized application and device management system in conformity with all health, safety, and environmental protection regulatory standards.
More on Michimich.com
Find out more about the Rhenium-SCT® at www.oncobeta.com
Follow us on social media:
LinkedIn: https://www.linkedin.com/company/oncobeta-gmbh/
Facebook: https://www.facebook.com/oncobeta/
Instagram: https://www.instagram.com/oncobeta_gmbh/
Forward-looking statements
This announcement includes forward-looking statements that involve risks, uncertainties, and other factors, many of which are outside of OncoBeta's control, and which could cause actual results to differ materially from the results discussed in the forward-looking statements. Forward-looking statements include statements concerning OncoBeta's plans, objectives, goals, future events, performance and/or other information that is not historical information. All such forward-looking statements are expressly qualified by these cautionary statements and any other cautionary statements which may accompany the forward-looking statements. OncoBeta® undertakes no obligation to publicly update or revise forward-looking statements to reflect subsequent events or circumstances after the date made, except as required by law.
*No pain reported during procedure.6
‡No direct skin contact or incisions.6
References
The EPIC-Skin Study (Efficacy of Personalised Irradiation with Rhenium-SCT for the Treatment of Non-Melanoma Skin Cancer) is designed to assess treatment efficacy and safety, as well as key patient-reported outcomes including quality of life, treatment comfort, and cosmetic results.1 The study is based on the clinically validated effect of the beta-emitter rhenium-188 in treating basal cell carcinoma (BCC) and squamous cell carcinoma (SCC).2
Treatment data is available for 184 adult patients (with 254 lesions) across 7 study centres in Australia, Austria, Germany, the United Kingdom, and South Africa. All participants had histologically confirmed stage I or II NMSC. The median patient age was 70.3 years (range 27–95 years), and 53.3% of participants were male. Patients had one (71.2%), two (19.6%), or three (9.2%) lesions treated, which were located on the head & neck (11.8%), trunk (11.8%), lower limbs (11.8%), or upper limbs (9.1%).1
Treatment & Methodology
Rhenium-SCT was administered as a single 50-Gy topical dose of rhenium-188 embedded in a resin applied via adhesive film to the lesion site. Efficacy was assessed using modified RECIST criteria, at 12 months follow-up. Quality of life was assessed using the Skin Cancer Index (SCI) at baseline, 6 months, and 12 months. Treatment comfort was assessed using a patient questionnaire, whilst patient- and clinician-evaluated cosmetic outcomes were determined using a visual analogue scale (VAS) grading of 0-10 (0 = very poor; 10 = no wound detectable). Safety was assessed via treatment-emergent adverse events (TEAEs) and CTCAE (Common Terminology Criteria for Adverse Events) grading.
Key 12-Month Results:1
- Overall response rate: 97.3%
- Complete response rate: 94.1%
- Partial response rate: 3.2%
- Mean improvement in quality of life: +10.55 points from baseline
- Pain/discomfort during treatment: None reported
- Cosmetic outcomes: Favourable results reported by both patients and clinicians
- Most common toxicity (12-month): Grade 1 hypopigmentation (60.4%)
- No CTCAE toxicities above Grade 2 observed at 12 months
These findings confirm that Rhenium-SCT®, administered in a single session, delivers sustained high efficacy and improved quality of life, with excellent cosmetic outcomes and minimal adverse effects.
More on Michimich.com
- Philharmonic hires new executive director
- Walker Farms, 50-Year-Old Florida Honey Business, Offered for $4M Sale
- David Oberman Debuts "Americana From Alabama" Project With The Release Of Two New Songs
- Century Fasteners de Mexico Awarded AS9120B and ISO9001:2015 Quality Certifications
- Colbert Packaging Invites Visitors to Booth #N-5476 at PACK EXPO
"Rhenium-SCT® has consistently demonstrated effectiveness and safety in prior studies," said Dr. Gerhard Dahlhoff, Medical Director at OncoBeta®. "This 12-month interim data further reinforces its value as a non-invasive, targeted treatment option for NMSC, particularly for patients seeking alternatives to surgery due to cosmetic or health-related concerns."
"The 12-month results from the EPIC-Skin Study represent a major milestone for OncoBeta®," added Shannon D. Brown III, CCO OncoBeta & Managing Director Europe. "These results support the efficacy, safety, and patient satisfaction associated with Rhenium-SCT®. These are the three foundational pillars of our patient-first vision, emphasising not only clinical outcomes, but also the patient experience in achieving them."
_
About the Rhenium-SCT® (Skin Cancer Therapy)
Non-melanoma skin cancer (NMSC) is the most common form of cancer in humans.3 The most common cause of NMSC is sun exposure, while other predisposing factors include genetic skin conditions and immunosuppressive diseases or treatments.4
The Rhenium-SCT is a painless*, non-invasive‡ therapy that provides aesthetic results, even in cases otherwise considered difficult to treat.1,5,6 The Rhenium-SCT utilises the radioisotope Rhenium-188 in an epidermal application with optimal properties for the treatment of NMSCs (non-melanoma skin cancers). The Rhenium-SCT is a precise, personalised therapy2 that is only applied to the area needed to treat without affecting the healthy tissue. The specially designed device ensures the Rhenium-SCT compound never comes in direct contact with the patient's skin and the application is safe and simple for the applying physician. Scar-free healing of the treated lesion area and the regeneration of healthy tissue occurs usually within a few weeks after treatment.6-8
About OncoBeta®
OncoBeta® with its headquarters located in Garching near Munich, Germany, is a privately held medical device company, specializing in the development and commercialization of state-of-the-art, innovative therapies. Since its foundation, OncoBeta® has concentrated its efforts on the development, regulatory approval(s) and commercialization of the epidermal radioisotope therapy Rhenium-SCT® (Skin Cancer Therapy), targeting non-melanoma skin cancers and keloid scars. OncoBeta® has perfected the customized application and device management system in conformity with all health, safety, and environmental protection regulatory standards.
More on Michimich.com
- Comerica Bank and Jackets for Jobs Join Forces to Support Local Teens This Homecoming Season with Free Dress Giveaway
- Men's Health Network Launches 2025 Prostate Cancer Awareness Month Campaign:
- Nashville International Chopin Competition Reflects on Global Summer, Unveils Fall & Winter Programs
- Phinge Invites Global Social Media Platforms & Major Brands To Join Netverse App-less Verified Platform To Reward & Safeguard Their Users & Customers
- Next-Generation Website Launch Highlights New Growth Phase Including $10 Million Acquisitions Plan for AI Powered Sports, Entertainment, Gaming Leader
Find out more about the Rhenium-SCT® at www.oncobeta.com
Follow us on social media:
LinkedIn: https://www.linkedin.com/company/oncobeta-gmbh/
Facebook: https://www.facebook.com/oncobeta/
Instagram: https://www.instagram.com/oncobeta_gmbh/
Forward-looking statements
This announcement includes forward-looking statements that involve risks, uncertainties, and other factors, many of which are outside of OncoBeta's control, and which could cause actual results to differ materially from the results discussed in the forward-looking statements. Forward-looking statements include statements concerning OncoBeta's plans, objectives, goals, future events, performance and/or other information that is not historical information. All such forward-looking statements are expressly qualified by these cautionary statements and any other cautionary statements which may accompany the forward-looking statements. OncoBeta® undertakes no obligation to publicly update or revise forward-looking statements to reflect subsequent events or circumstances after the date made, except as required by law.
*No pain reported during procedure.6
‡No direct skin contact or incisions.6
References
- Cardaci G, et al. Adv Radiat Oncol. 2025;10(7):101802.
- Cipriani C, et al. J Dermatol Treat. 2022;33(2):969–975. Epub 22 Jul 2020.
- Ciążyńska M, et al. Sci Rep. 2021;11(1):4337.
- Cancer.net. Skin Cancer (Non-Melanoma): Risk Factors and Prevention. February 2022. https://www.cancer.net/cancer-types/skin-cancer-non-melanoma/risk-factors-and-prevention (accessed June 2025).
- Tietze JK, et al. Clin Nuc Med. 2023;48(10):869–876.
- Castellucci P, et al. Eur J Nucl Med Mol Imaging. 2021;48(5):1511–1521.
- Cipriani C, et al. Int J Nucl Med. 2017;114–112.
- Sedda AF, et al. Clin Exp Dermatol. 2008;33(6):745–749.
Source: OncoBeta GmbH
Filed Under: Health
0 Comments
Latest on Michimich.com
- QFIA and Aparx Partner to Build Cross-Border Compliance Practice Platform, Second Course Officially Launches
- Fonteviva® Confirms U.S. Entry; E-Refer Sourcing Secures 10-Year Exclusive U.S. Importation & Distribution Rights
- Floridians Educated on Mental Health Abuses at the "Psychiatry: An Industry of Death" Traveling Exhibit in Orlando
- Nebuvex Exchange Announces Completion of Beta Testing, Prepares Q3 2025 US Market Launch
- Transform Your Eyes: How to Lift Away Drooping or Hooded Eyelids
- The Other 95%: A Groundbreaking New Book Unlocks the Hidden Power of the Human Mind
- The Learning Circle Childcare Centre – South Surrey Campus Currently Enrolling for September & Fall
- New Ownership, Same Heart: Jimmy & Jennifer Jhanda Take The Reins At Primos
- THE LEAGUE: Where Basketball Meets Culture in the Heart of LA — Played at The Surgeon
- Integris Composites Joins Pacific Future Forum in Tokyo
- Doberman Pup Explores Off-Grid Island in New Outdoor Adventure Release
- BusinessRate Selects New Jersey Therapy & Life Coaching as Best Couselors
- IRL Investigations Combines Decades of Experience with Modern Digital Expertise
- New Leadership Model – Never Fire Anyone – Released Today
- AureaVault Launches U.S.-Licensed Cryptocurrency Exchange with Enhanced Security Features
- IOTAP Named to 2025 Inc. 5000 List of America's Fastest-Growing Private Companies
- Lineus Medical and Venture Medical Sign New Zealand Distribution Agreement
- Black Plumbing Expands to Cleburne, TX, Bringing Over 30 Years of Trusted Plumbing Service
- Rocket Companies CFO Brian Brown to Present at Barclays Global Financial Services Conference
- $5 - $20 Million in Sales for 2026; $25 - $40 Million for 2027 Projected with NASA Agreements; New MOU Signed to Improve Solar Tech in Space